In the world of tissue engineering, collagen scaffolds have become recognized as biological templates with which damaged tissue can be regenerated. In this context, regeneration would mean control over the behaviours of multiple cell types, for example, encouraging the invasion of the appropriate precursor cells, while at the same time, also preventing the growth of unwanted cells. However, the movement of cells through a scaffold depends on the cell type, and understanding this relationship is key to creating a scaffold capable of guiding the regeneration of complex tissues.
Jennifer Ashworth and co-workers describe how this cell type dependency can be leveraged to establish cellular sieving, wherein the scaffolds physically separate cells according to their invasive capacity, by playing around with two structural parameters: the pore size and percolation diameter (the diameter of the largest object that can pass through the scaffold). In their study, the invasion behaviour of cells from three different sources with distinct tissue engineering applications were analysed: human primary fibroblasts, the MC3T3 mouse osteoprogenitor cell line, and the HT1080 cell line from human fibrosarcomas.
Four scaffolds with successively increasing pore sizes and percolation diameters, measured via X‐ray micro‐computed tomography, were developed by freeze‐drying type I collagen suspensions under controlled environments.
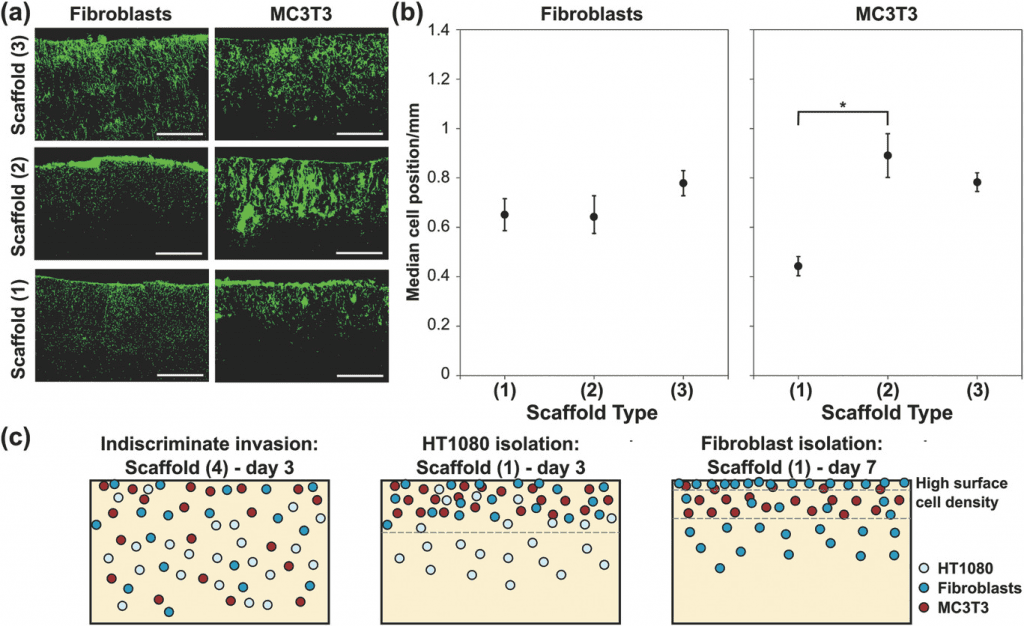
After seeding the cultured cells onto the scaffolds, the invasion distance was calculated on the basis of the fluorescent intensity profile. The results showed that the scaffolds with the largest pore sizes and percolation diameters did not provide any substantial structural barrier to the invasion of all cell types. With the sequential decrease in pore size and percolation diameter, the scaffold acted as a cellular sieve, allowing the successive invasion of HT1080 cells, followed by primary fibroblasts; the majority of the MC3T3 cells were trapped close to the scaffold surface.
The authors believe that the findings of this study could have important implications in developing bioassays intended for specific applications, models of cancer metastasis, and biological materials for the regeneration of complex tissues in vivo.