Nitrous oxide (N2O ) is a p otential greenhouse gase with 310 times the global warming potential of carbon dioxide (CO2). In addition, nitrous oxide (N2O) has a long atmospheric lifetime, of about 120 years. It has been estimated that doubling N2O concentration could result in an up to 12% decrease in the total stratospheric ozone.
otential greenhouse gase with 310 times the global warming potential of carbon dioxide (CO2). In addition, nitrous oxide (N2O) has a long atmospheric lifetime, of about 120 years. It has been estimated that doubling N2O concentration could result in an up to 12% decrease in the total stratospheric ozone.
Traditional approaches such as thermo-catalytic methods are not very effective in handling large volumes of N2O. However, N2O may be used as a potential oxidant if it is decomposed into N2 and O.
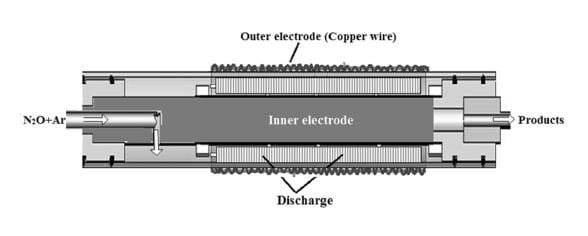
Now, new research from a group based at Energy and Environmental Research Lab of the Indian Institute of Technology Hyderabad deals with the application of nonthermal plasma operated in dielectric barrier discharge for the direct decomposition of N2O into its constituent elements. The team studied the influence of various parameters like discharge gap, input power, residence time and nitrous oxide concentration to achieve the best decomposition. Typical results indicated that N2O decomposition may be efficient at high residence time and low concentrations. However, from the energy point of view high concentration and low residence times are preferred.
An additional interesting observation is that packing the discharge volume with dielectric materials (ceramic, glass and Al2O3 beads) improved the conversion, probably due to changes in the discharge characteristics. Under the same experimental conditions, the effect of the dielectric materials followed the order: ceramic>beads>glass beads>Al2O3 bead>no packing. These results may lead to a new efficient way to reduce the emission of nitrous oxide in the atmosphere.