DNA replication is executed by the so-called replisome – a multicomponent molecular machine responsible for unwinding and then duplicating the DNA. It was long believed that DNA replication follows a single highly coordinated and reproducible sequence of molecular events. However, single-molecule analyses of the replication process have begun to challenge this view.
Taking into account these single-molecule studies, Matthias Scherr, Barbara Safaric and Karl Duderstadt review our current understanding of DNA replication. In their ‘Think again’ article published in BioEssays, they point out that DNA replication is a very dynamic process characterized by an exchange of core components and by stochastic sampling of multiple molecular pathways.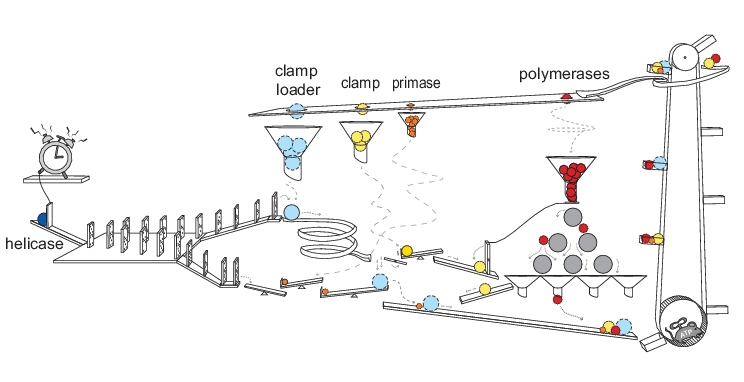
The authors first discuss various single-molecule approaches used to studying replisome function. Magnetic and optical tweezers, for example, work by stretching a DNA molecule and then monitoring the resulting length changes. These methods have been used to study DNA unwinding by helicases, the kinetics of nucleotide incorporation during synthesis as well as DNA looping dynamics. Fluorescence imaging techniques are also being used to study DNA replication. These methods have the advantage of additionally providing information about the spatial and temporal organization of individual components within the large replisome complex. Fluorescence resonance energy transfer (FRET) and co-localization single-molecule spectroscopy (CoSMoS) are two examples of such methods.
Next, the authors present current working models for bacterial replication. During DNA replication, the polymerase synthesizes DNA in the 5’ to 3’ direction in both strands. This directionality leads to the leading strand being synthesized continuously whereas the lagging strand is replicated discontinuously. Different models are discussed how daughter-strand synthesis remains temporally coordinated despite this discrepancy. Interestingly, recent experimental evidence suggests that leading- and lagging-strand synthesis are not functionally coupled but that instead stochastic events are at play.
And finally, the authors discuss our current view of eukaryotic DNA replication. Here, different models for helicase loading and activation are presented. Additionally, the role of the various polymerases and their dynamic exchange is discussed. Overall, the recently revealed plasticity and dynamic nature of DNA replication provides adaptability and flexibility – an important feature for conferring robustness in case of sudden changes in cellular conditions.