Biomarkers are disease-related cellular or molecular changes in the human body, and their reliable detection is of utmost importance for clinical diagnostic and therapeutic applications. Common disease biomarkers include genes or genetic mutations, proteins, and single-cells found in disease-related tissue as well as biofluids such as blood, urine, and sputum, among others. Unfortunately for many diseases, low abundance of biomarkers in human samples and low sample volumes render standard bulk platforms like 96-well plates ineffective for reliable detection or screening. Further, most standard bulk protocols are largely incapable of detecting biomarkers directly from clinical patient samples, and therefore require complex multi-step sample preparation methods that can be time consuming, and in a clinical setting, can contribute to delayed patient care. Therefore, there exists a need for sensitive, rapid, automatable, and high-throughput sample-to-answer platforms for detection and screening of disease biomarkers.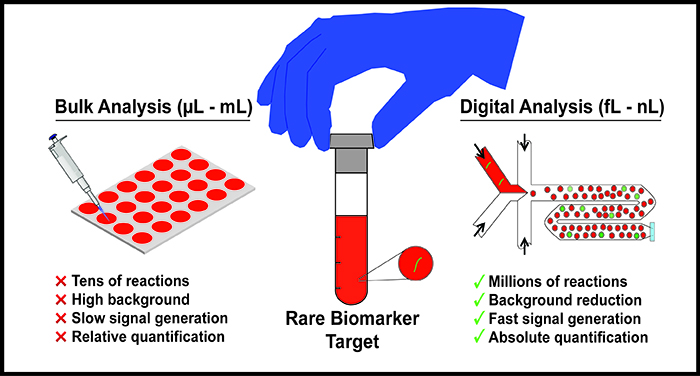
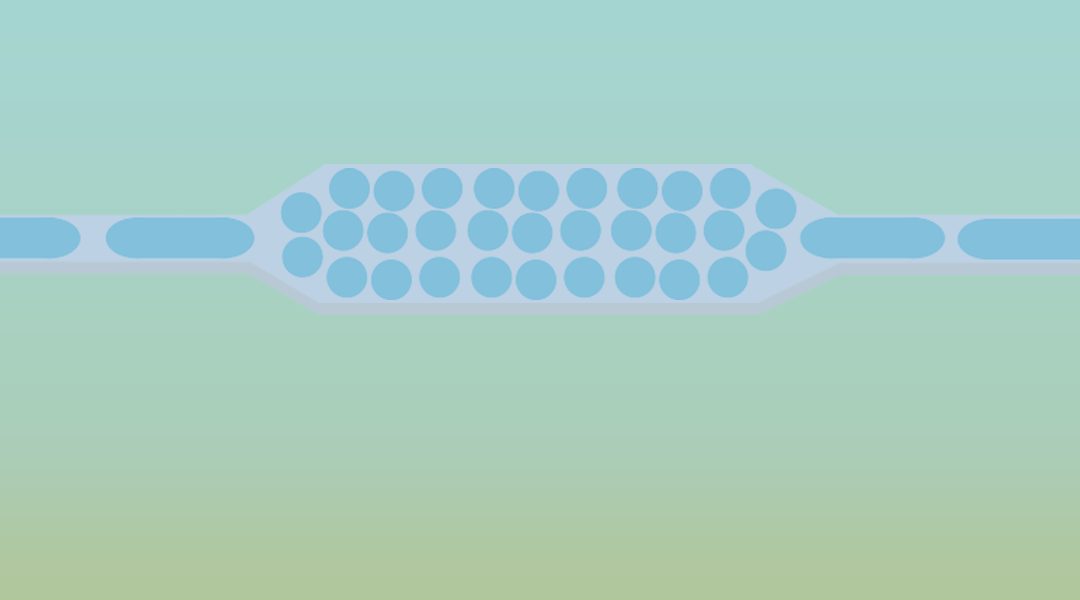
Due to its ability to precisely manipulate fluids at small volumes, the field of microfluidics has presented useful tools for biomarker detection. In particular, digitization of bulk samples into a large number of isolated small volumes (fL-nL) via droplet microfluidic technology offers a promising solution for high-sensitivity and high-throughput biomarker detection. The significant reduction in volume achieved via digitization of biomarkers in droplets facilitates an equivalent reduction in assay background and a drastic increase in the local concentration of the biomarker target. This in turn increases the signal to background ratio from each isolated reaction and consequently increases the overall sensitivity and speed of the assay. Further, running potentially millions of single-biomarker reactions from an entire sample in parallel can not only help detect rare targets but it can also enhance multiplexed sample screening. Over the last decade, a wide array of microfluidic droplet platforms have been developed in order to detect, quantify, and screen nucleic acids, proteins, and single-cells from human-derived samples for diseases ranging from bacterial infections to cancer. For example, digitization of single-cells of pathogenic bacteria from human biofluids (eg: blood) has allowed for rapid diagnostic tests of pathogen identity and antimicrobial susceptibility, in order to shorten the turnaround time in the clinical microbiological diagnostic workflow. Likewise, commercial droplet platforms (eg: BioRad’s ddPCR) have provided a turnkey tool for detection and quantification of cancer-specific mutations from human genetic samples with single-molecule sensitivity.
The potential of rapid diagnostics and screening has led to burgeoning interest in droplets, as it is increasingly used to detect novel disease biomarker targets. Yet, most droplet platforms rely extensively on fragmented workflows that require off-chip steps for sample purification and droplet incubation, and as a result, clinical implementation of a fully integrated sample-in-answer-out droplet platform is yet to be realized. In achieving this goal, seamless integration of sample preparation technologies as well as automation of platforms from patient sample to clinically-actionable answer remain critical components that can render droplet-based platforms useful in the clinical setting in the near future.
Read more in a recent review article in WIREs Nanomedicine and Nanobiotechnology.
Kindly contributed by the Authors