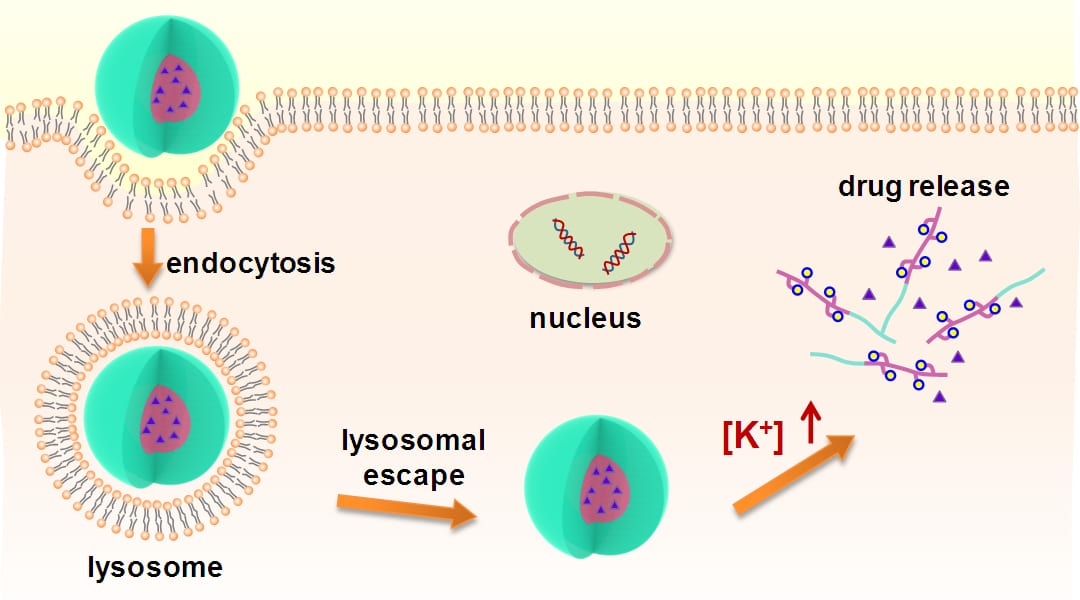
Drug nanocarriers have significant potential to improve cancer treatment and specifically polymeric micelles show unique advantages as nanocarriers for encapsulating poorly water-soluble drugs. Especially, stimuli-responsive micelles that can respond to various environmental stimuli such as temperature, pH and light, can achieve targeted drug delivery and controlled drug release.
In the human body, the K+ concentration in intracellular liquid (140 ~ 150 mM) is about 30 times higher than that in extracellular liquid (3.5 ~ 5.5 mM). Such a significant K+ difference can be utilized as a trigger for controlled drug release in vivo. In a recent paper from Molecular Bioscience, Prof. Ju and her co-workers at School of Chemical Engineering in Sichuan University have developed a novel type of K+-responsive micelle with a controlled drug release triggered by intracellular high K+ concentration. The micellar structure is composed of a hydrophilic shell and a K+-responsive core. The micelles show low toxicity to normal cells in a certain concentration range. It was shown that the drug encapsulated in the micelles could be delivered and specifically released inside the cells. In response to the high K+ concentration of 150 mM in intracellular liquid, the drug-loaded micelles display a significantly accelerated drug release rate, compared with that in extracellular liquid with a low K+ concentration of 5 mM. Therefore, the proposed K+-responsive micelles provide a novel mode for designing new-generation smart nanocarriers for targeted intracellular drug delivery and are highly potential in the field of targeted therapy.