The goal of securing a pollution-free environment and meeting future energy demands has motivated the development of renewable energy sources to replace conventional fuels. Electrocatalytic approaches such as those depending on the oxygen evolution reaction (OER) along with the hydrogen evolution reaction (HER) for water splitting have attracted great attention for electrochemical energy conversion and storage as renewable energy devices.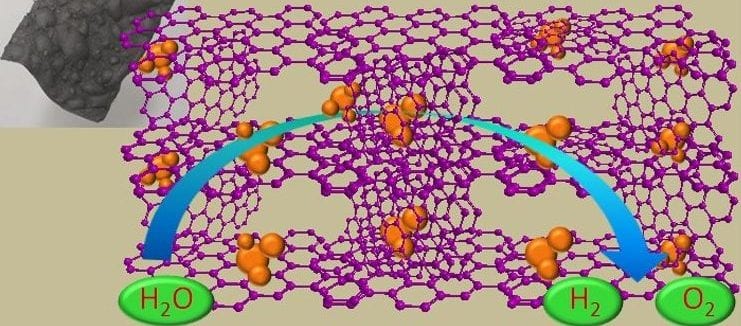
The present challenge is to develop an efficient, durable, and low-cost bifunctional electrocatalyst for these reactions to help to meet the need for environmentally friendly renewable energy systems. The high cost and scarcity associated with platinum and ruthenium based electrocatalysts have greatly hindered any extensive application for them in water splitting. Accordingly, developing an efficient alternative electrocatalyst by using earth-abundant inexpensive elements to substitute for those noble metal catalysts is of great significance.
Metal/metal oxide-incorporated carbon nanocomposites have attracted great attention as alternative bifunctional electrocatalytic materials for efficient water splitting. Most of the bifunctional electrocatalysts developed so far, however, are powdery and require conductive as well as binding components for electrode fabrication, which makes the process time-consuming and complicated for large-scale production.
In recent work reported by Konstantin Konstantinov and co-workers at the University of Wollongong, in collaboration with researchers at The University of Sydney, Australia cobalt/cobalt oxide nanoparticles are incorporated in a graphene film to exploit the liquid crystal (LC) behavior of graphene oxide (GO) sheets. The large number of functional groups of liquid-crystal graphene oxide (LC GO) facilitates the interaction with cobalt nitrate in an aqueous medium, and the self-assembly characteristic of LC GO favors the formation of free-standing films at low temperature. Under thermal treatment (600 °C) in an inert atmosphere, the graphene oxide/cobalt salt composite is transformed into cobalt/cobalt oxide (Co/Co3O4) nanoparticles embedded in a free-standing, three-dimensional (3D) macrostructured framework of reduced graphene oxide film (Co/Co3O4-Gr). The conductive 3D network makes it possible to access the entire area as well as the Co/Co3O4 nanoparticles in the electrodes to achieve efficient electrocatalytic activity towards the OER and HER to allow efficient water splitting from a single electrode.
This article is part of a Special Issue on “Graphene Oxide Liquid Crystals” published in Particle & Particle Systems Characterization. Click here to go directly to the table of contents http://onlinelibrary.wiley.com/doi/10.1002/ppsc.v34.9/issuetoc and here to read the Editorial http://onlinelibrary.wiley.com/doi/10.1002/ppsc.201700261/full contributed by the guest editors Kyung Eun Lee and Sang Ouk Kim.
















