 The building blocks for nanoparticles include metals, lipids, chemical and biological polymers. Biological NPs are less toxic and expensive compared to their metallic and chemical counterparts, making them desirable candidates for the development of powerful nanotherapeutics.
The building blocks for nanoparticles include metals, lipids, chemical and biological polymers. Biological NPs are less toxic and expensive compared to their metallic and chemical counterparts, making them desirable candidates for the development of powerful nanotherapeutics.
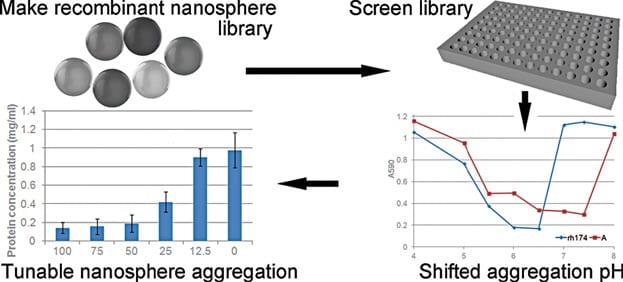
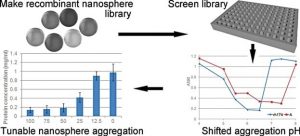
In new work, Johan Bonde and Leif Bülow investigated amelogenin as a new possible candidate to create protein-based recombinant NPs. Amelogenin is involved in the formation and biomineralization of dental enamel during tooth development. Numerous studies demonstrated that a recombinant amelogenin spontaneously assemble into supramolecular quasi-spherical aggregates, or “nanospheres”. The natural ability of a recombinant amelogenin to form NPs makes it a perfect material for nanomedical applications.
The self-assembly of amelogenin into nanospheres is a highly pH dependent process requiring directed engineering of this protein to fine tune and control its solubility, which is crucial for certain drug delivery applications where the right pH value is a key for their efficiency. In this study, the authors created an amelogenin mutant library by use of random mutagenesis. Fifty recombinant amelogenin mutants were purified and characterized based on their solubility/aggregation profiles under different pH conditions. Several of these mutants were able to form uniform nanospheres, ranging from 30 to 60 nm in hydrodynamic diameter. The mutants aggregated at a different pH compared to wild-type amelogenin. At pH 7.4, some mutants formed soluble nanospheres, while others generated nanosphere aggregates. Depending on their solubility and by creating heterogenous nanospheres built from mutant and wild-type variants, amelogenins can be put to different practical uses including controlled drug release at target sites.