The past few years have marked a substantial paradigm shift in design criteria for modern synthetic biomaterials, fully integrating principles from cell and molecular biology. New generations of synthetic biomaterials are being developed for use as three-dimensional extracellular microenvironments to mimic the regulatory characteristics of natural extracellular matrices for therapeutic applications.
The past few years have marked a substantial paradigm shift in design criteria for modern synthetic biomaterials, fully integrating principles from cell and molecular biology. New generations of synthetic biomaterials are being developed for use as three-dimensional extracellular microenvironments to mimic the regulatory characteristics of natural extracellular matrices for therapeutic applications.
Considering a specific example, current strategies for jaw reconstruction require multiple procedures, first to repair the bone defect to offer sufficient support, and then to place the tooth implant. The entire procedure can be painful and time-consuming, and the desired functional repair can be achieved only when both steps are successful. The ability to engineer combined tooth and bone constructs, which would grow in a coordinated fashion with the surrounding tissues, could potentially improve the clinical outcomes, and also reduce patient suffering.
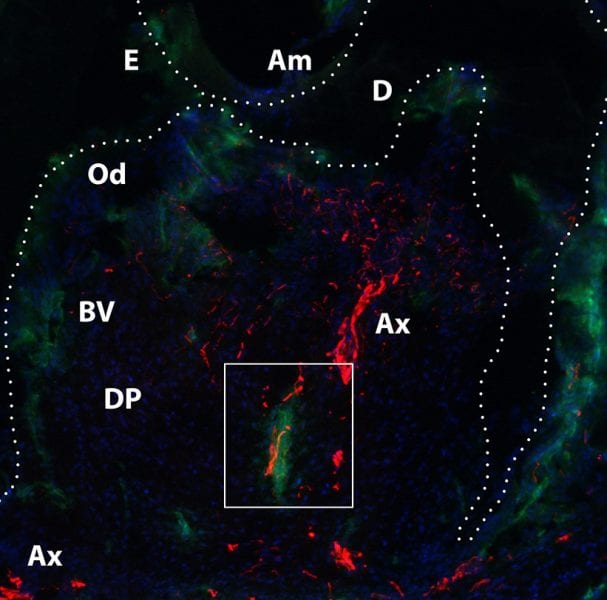
Now, in new work, a French research group report not only the first demonstration of a unique nanofibrous active implant for bone-tooth unit regeneration but also the innervation of this bioengineered tooth. Tooth innervation must be achieved to obtain a fully functional organ.