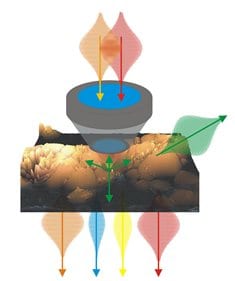
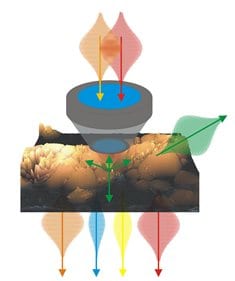
Multimodal nonlinear microscopy has matured to a key imaging modality in life science and biomedicine. It offers label-free visualization of tissue structure and chemical composition, high depth penetration, intrinsic 3D sectioning, diffraction limited resolution and low phototoxicity.
 In the developed countries, the major cause of death are lifestyle-induced diseases such as cardiovascular diseases and cancer. In their early stages, they are hard to diagnose, and in their late states very difficult to treat. Current biomedical research aims at earlier detection, which requires novel techniques for visualizing subtle changes, e.g., DNA damage at the molecular level. Label-free imaging methods are preferred for in vivo applications, since they are more robust to variations and rely on the direct detection of marker molecules.
In the developed countries, the major cause of death are lifestyle-induced diseases such as cardiovascular diseases and cancer. In their early stages, they are hard to diagnose, and in their late states very difficult to treat. Current biomedical research aims at earlier detection, which requires novel techniques for visualizing subtle changes, e.g., DNA damage at the molecular level. Label-free imaging methods are preferred for in vivo applications, since they are more robust to variations and rely on the direct detection of marker molecules.
In this context, nonlinear imaging provides several advantages: high depth penetration due to NIR laser, intrinsic 3D sectioning and 3D resolution due to the spatial confinement of the signal to the laser focus, a multitude of endogenous molecular markers as well as process parameters provide chemical contrast, low phototoxicity allows the investigation of living processes in the native environment without perturbance.
During the last years, some realizations of nonlinear microscopy have matured into routine imaging tools in the life sciences. However, in order to unravel the complex mechanisms of disease progression and metabolism, the distribution of multiple relevant markers need to be studied. Furthermore, the inspection of larger tissue volumes with high resolution would be desirable. In order to achieve this, multiple approaches have been recently developed by combining several imaging modalities to so-called multimodal or multicontrast imaging platforms. They allow for detecting multiple contrast agents or provide an extraordinary size range for investigation. The use of multiple contrast mechanisms opens up a wealth of synergies.
In a review article, Jürgen Popp and a team from the University of Jena (Germany) summarize recent advances in the field of multimodal nonlinear imaging in the biomedical sciences regarding the methodology, such as contrast mechanisms and signal characteristics used for contrast generation as well as novel image processing approaches. In addition, they report on technologic developments emphasizing improvements in penetration depth, imaging speed, spatial resolution and nonlinear labeling strategies. The last part of their article focuses on recent applications in life science fundamental research and biomedical diagnostics as well as future clinical applications.
The combination of multiple contrast mechanisms is used in two research areas. The first is the generation of chemical maps of complex tissues. Multimodal nonlinear microscopy allows investigating the structure of single cells, tissues and organs as well as monitoring structural and chemical changes related to disease progression. Second, multimodal nonlinear imaging is applied to investigate dynamic living processes. Multimodal nonlinear imaging is used to non-invasively investigate complex dynamic mechanisms, e.g., of signal transduction, nutrient transport, drug delivery and disease progression. Even though for selected biomedical applications a single nonlinear modality may be sufficient, e.g., SHG for cell sorting of pluripotent stem cell derived cardiomyocytes in flow cytometry,156 the analysis of further characteristics of the emitted nonlinear signals beside the intensity provides additional insight, e.g., in molecular ordering and orientation as shown in case of SHG. Other applications significantly benefit from the multimodal combination of several imaging modalities due to the enlarged number of available endogenous molecular markers, e.g. for label-free tissue histopathology.
To achieve further improvements, research focuses the combination of multimodal nonlinear imaging with imaging methods, which enable investigation of even larger intact tissue volumes, e.g., OCT or even MRI. For routine applications in clinics, especially technological improvements are required for miniaturization, improvement of the ease of handling and automated data processing and extraction of relevant information. An important step toward this goal is the modification of standard clinical endoscopes for multimodal nonlinear imaging suited for in vivo applications.