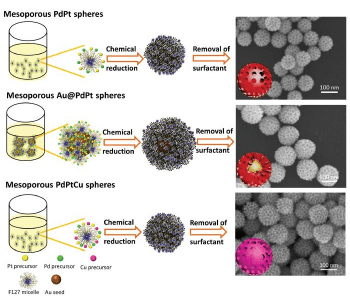
Bo Jiang and colleagues developed a facile, green, and tunable synthesis for mesoporous spheres consisting of multiple noble metals (Pt, Pd, Cu, Ag). These potential catalysts for a variety of applications, including fuel cells, can be fabricated in aqueous solution with low concentrations of a surfactant (F127). While the surfactant directs the uniform size and shape of the mesopores, the sphere size can be easily controlled by the ratio of the used Pt complexes. The synthesized trimetallic Au@PdPt and PdPtCu spheres catalyze the oxidation of methanol more effectively and with higher durability than dendritic Pt spheres or commercial Pd black.
Bo Jiang and colleagues developed a facile, green, and tunable synthesis for mesoporous spheres consisting of multiple noble metals (Pt, Pd, Cu, Ag). These potential catalysts for a variety of applications, including fuel cells, can be fabricated in aqueous solution with low concentrations of a surfactant (F127). While the surfactant directs the uniform size and shape of the mesopores, the sphere size can be easily controlled by the ratio of the used Pt complexes. The synthesized trimetallic Au@PdPt and PdPtCu spheres catalyze the oxidation of methanol more effectively and with higher durability than dendritic Pt spheres or commercial Pd black.
The size of PdPt spheres can be increased by increasing the amount of H2PtCl4 during the synthesis, which results in a slower growth rate of Pt on the Pd nucleus. This leads to spheres with diameters from 46 nm for no H2PtCl6 to 176 nm for 100% H2PtCl6. The size and shape of the mesopores is not affected by changing the sphere size. The single components in the PdPtCu spheres, which show diameters of 110 nm, are evenly distributed. In contrast, Ag nanoparticles are coated with PdPt in Ag@PdPt spheres with a diameter of 150 nm.
 Introducing multiple noble metals, such as Pd and Pt, into catalysts leads to interactions, which can enhance catalytic activity. In this case, Pt–Pd alloy structures at the internal surface of the spheres reduce the electronic binding energy. The mesopores are also very important for the catalytic performance because they have a large number of kink and step sites on their surface. Additionally, the ultra-large pore size and the uniform pore distribution accelerate the diffusion of reactants and products.
Introducing multiple noble metals, such as Pd and Pt, into catalysts leads to interactions, which can enhance catalytic activity. In this case, Pt–Pd alloy structures at the internal surface of the spheres reduce the electronic binding energy. The mesopores are also very important for the catalytic performance because they have a large number of kink and step sites on their surface. Additionally, the ultra-large pore size and the uniform pore distribution accelerate the diffusion of reactants and products.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.