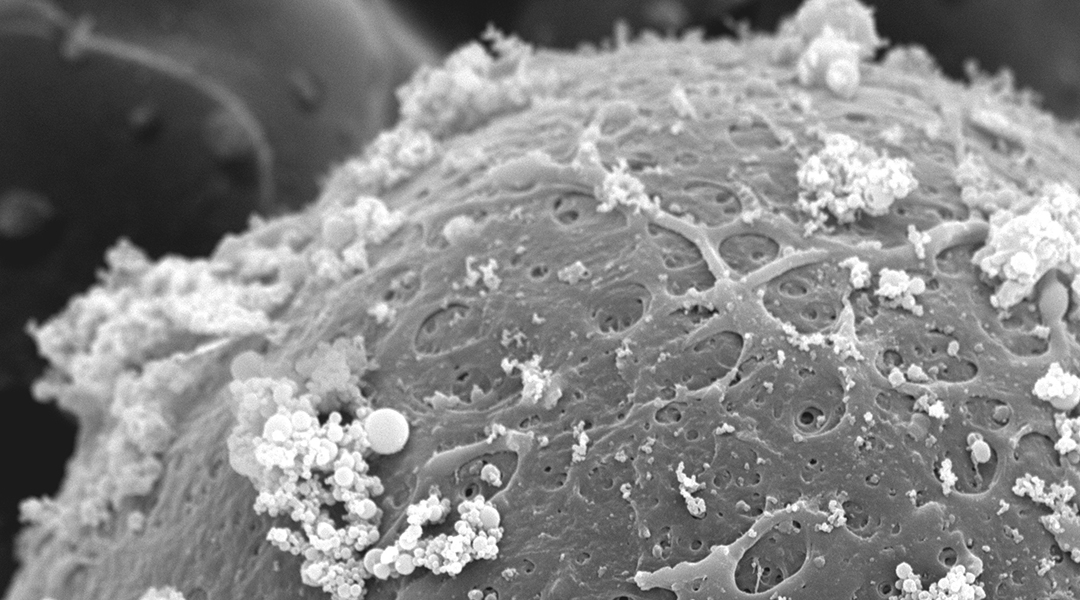
Eggs and embryos are the very first spark of life, but to nurture a full flame from that fragile flicker requires great delicacy. Many assisted reproduction technologies rely on selecting and manipulating these cells to safely embed them in place for growth, but this necessary interference raises the risks of disrupting healthy development.
To get around this, researchers have developed a way to control this movement in the lab without direct touch, by introducing nanoparticles that settle on the material surrounding the egg and can be pushed or pulled remotely through a magnetic field.
“Nanoparticles had never been used as a component for the manipulation of eggs and embryos before,” said María Jiménez-Movilla from the University of Murcia and lead author of the study published in the journal Advanced Science.
The result is a new and unique form of remote control of eggs in lab setups that could transform what is possible with fertility treatments in the future.
Magnetic attraction
The idea, says Jiménez-Movilla, arose as a challenge to design a strategy in which an egg could be displaced through a capillary, mimicking the natural path it takes in the duct called the oviduct, where fertilization occurs.
Movement along the capillary is achieved by fusing the nanoparticles to a protein present in the fallopian tubes during fertilization and development. In the body, this protein binds to embryos as they make their way towards the uterus.
“In our method, this protein acts as a natural bridge between nanoparticles and the matrix surrounding eggs and embryos, enabling precise and non-invasive manipulation,” said Jiménez-Movilla. Creating a complex of nanoparticles and protein that fix to the outside of early-stage embryos gives researchers a way to control their progress without risking physical contact.
The method grants remote control, but could the presence of the nanoparticles themselves impact development at all? “The biggest challenge has been to demonstrate through different approaches that the application of the method is entirely harmless and does not affect reproductive processes or the reproductive quality of gametes or embryos,” said Jiménez-Movilla.
This involved evaluating each stage of the process individually, from sperm and egg meeting to embryo implantation in the uterus, at both the cellular level and in terms of eventual impact on offspring. They discovered that rabbit embryos bound by the nanoparticles successfully developed to mature rabbits at the normal rates — a very promising sign for the method’s safety.
Allowing healthy development was the fundamental goal for the team, but they found a surprising bonus to the technique as well: The method appears to filter out eggs that would not be viable for development.
“We were surprised that when we incubated nanoparticles with eggs and applied the magnetic field to retain them, the small proportion of eggs that were not retained corresponded to immature and non-competent eggs for fertilization,” said Jiménez-Movilla.
The magnetic nanoparticles seem to exclusively bind to competent eggs, further boosting their utility in any procedure designed to identify and support eggs with the greatest potential for full development.
Next steps
These microscopic pushes could move the whole field forward. “The methodology itself represents a paradigm shift in the manipulation of eggs and embryos,” said Jiménez-Movilla. “It may require technological effort, but could result in numerous applications advancing substantially in reproductive biotechnology across different mammalian species.”
The team aim to create improved lab-based reproduction models that more closely mimic natural processes happening inside the body. Specifically, moving eggs and embryos through structures that replicate the characteristics of the fallopian tube would bring fertilization techniques closer to natural conditions, and so improve outcomes.
Navigating the movement of these types of cells immersed in fluid through these structures has long presented a biotechnological challenge, which this method overcomes, providing a platform for research and perhaps, ultimately, improving prospects for the millions of people with fertility difficulties.
“We advocate for the advancement of […] reproduction techniques towards more sophisticated biotechnological methods, such as microfluidic technologies (i.e., a comprehensive on-chip IVF platform), capable of replicating natural fertilization and early embryonic development processes,” added Jiménez-Movilla.
Improving the capacity for subtle, precise, and gentle control of this most vulnerable material in laboratory settings will ultimately help researchers and doctors guide the development of life, and the growth of new families.
Reference: María Jiménez-Movilla, Francisco Alberto García-Vázquez, et al., Magnetic-Assisted Control of Eggs and Embryos via Zona Pellucida-Linked Nanoparticles, Advanced Science (2024). DOI: 10.1002/advs.202306901