Reliable, readily available tissue engineered organs, which can be used to regenerate or replace irreversibly damaged organs, are a significant unmet clinical need. Ideal tissue engineering approaches require artificial scaffolds to be tailor-made to mimic physiological environments of interest. This enables cells to perceive the artificial scaffolds as their natural environment to restore the physiological function of the damaged tissue.
Reliable, readily available tissue engineered organs, which can be used to regenerate or replace irreversibly damaged organs, are a significant unmet clinical need. Ideal tissue engineering approaches require artificial scaffolds to be tailor-made to mimic physiological environments of interest. This enables cells to perceive the artificial scaffolds as their natural environment to restore the physiological function of the damaged tissue.
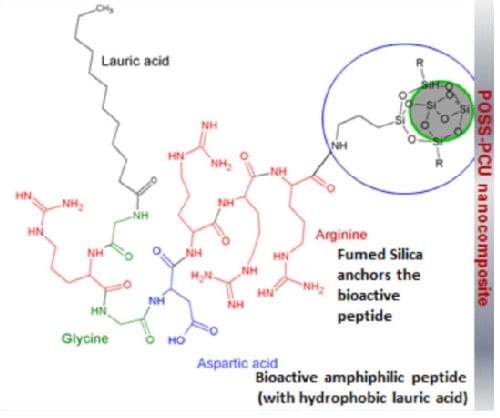
Alexander M. Seifalian et al. have functionalised fumed silica nanoparticles (FSN), to have ‘tethering’ proteins and link bioactive groups to induce biomimicry. A significant advantage of the use of these nanoparticles for the modification of the main scaffold material includes its ability to be fabricated with the state-of-the-art methodology with greater flexibility. This study uses RGD peptide, tethered to functionalised FSN to capture progenitor stem cells from circulating blood in the cardiovascular system, which has a layer of endothelial cells (ECs). RGD is an active protein component of the natural cellular- bimolecular environment. Adhered stem cells are known to differentiate in to functional ECs, from shear stress of blood.
Since this study, the principle presented in this article of using functionalized FSN is rapidly implemented in a range of surgical implants currently being developed. These include synthetic paediatric trachea, ear and nasal constructs, nitric oxide activators for cardiovascular implants and also for cell-specific antibody attachment in coronary artery stents, and bypass graft undergoing clinical trials. Therefore FSN mediated scaffold functionalisation, can be potentially adapted to induce cellular biomimicry in the development of a range of human organs.