 The development of commercial lithium-ion batteries (LIBs) with a graphite anode and LiCoO2 cathode is still a key bottleneck to meet current power demands, in particular those from portable electronics and electrical vehicles. Furthermore, development of high-performance LIBs with ultrafast charging /discharging rates and ultralong cycle life is highly demanded, and would significantly advance the next generation of energy storage technology. Normally, it takes hours for a battery to recharge. Rapid charging within a few minutes would be very promising. However, when batteries are charged at a high rate, a dramatic reduction in energy (capacity) and cycle life is usually observed.
The development of commercial lithium-ion batteries (LIBs) with a graphite anode and LiCoO2 cathode is still a key bottleneck to meet current power demands, in particular those from portable electronics and electrical vehicles. Furthermore, development of high-performance LIBs with ultrafast charging /discharging rates and ultralong cycle life is highly demanded, and would significantly advance the next generation of energy storage technology. Normally, it takes hours for a battery to recharge. Rapid charging within a few minutes would be very promising. However, when batteries are charged at a high rate, a dramatic reduction in energy (capacity) and cycle life is usually observed.
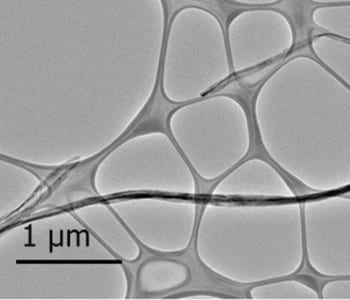
It is known that a stirring process can provide homogeneous mixing of reactants in solution, increasing reaction rate as well as maintaining reaction conditions such as temperature and concentration. By integrating this stirring technique into a traditional hydrothermal reaction, a team led by Prof. Xiaodong Chen at Nanyang Technological University in Singapore has produced longer TiO2 nanotubes – with lengths up to tens of micrometers (about twice) those of conventional nanotubes – and demonstrated their use in ultrafast recharging. The formation of this elongated nanotubular structure is due, the researchers believe, to the improvement of diffusion and chemical reaction rates under mechanical stirring, and the nanotubes also bend due to shear force. Based on this bent, elongated structure, the group built a robust three-dimensional network architectureof TiO2(B) nanotubes for ultrafast charging batteries. The devices demonstrated high-rate charging-discharging (< 3 min per charging, 8.4 A/g) for more than 10,000 cycles with a superior capacity (ca. 114 mAh g−1). This is equivalent to a product lifetime of more than 25 years (assuming one charge per day). The results were recently published in Advanced Materials.
Furthermore, the team have solved a general problem in current battery systems by making use of their unique stirring hydrothermal method. Currently, fundamental understanding of structure-performance relationships is still limited owing to the wide use of additives (e.g., polymeric binders and conductive agent) in conventional battery construction, which greatly alters the overall LIB performance. The Nanyang group developed an additive-free battery configuration that allow them to precisely elucidate the correlation of aspect ratio in nanotubular structure to their electrochemical performance. This work has just been published in Angew. Chem. Int. Ed. The additive-free concept is achieved by the development of viscous TiO2-based nanotubular materials through the stirring hydrothermal approach with good adhesion on the current collector; by tuning the stirring rate, different nanotube aspect ratios can be obtained for performance evaluation..
When such gel-like nanotubes are used to fabricate additive-free electrodes, the LIB performance can be modulated by changing the nanotube aspect ratio. The battery performance at high charge/discharge rates is dramatically boosted when the aspect ratio is increased, due to the optimization of electronic/ionic transport properties within the electrode materials. The proof-of-concept anatase TiO2 LIBs made from nanotubes with an aspect ratio of 265 can retain more than 86% of their initial capacity over 6000 cycles at a high rate of 30 C (2 min). This is equivalent to a product lifetime of about 20 years (assuming one charge per day). Such devices with supercapacitor-like rate performance and battery-like capacity herald a new paradigm for energy storage systems, the team believe, and they are currently taking further steps to scale up and commericalize this ultrafast battery product.