Traditionally, studies on cells have been performed by growing freshly isolated cells or immortalized cell lines in 2D culture dishes.
However, a major limitation of 2D culture is that it poorly mimics the environment of cells in the body. This is to a great extent due to the lack of cell–cell and cell–extracellular matrix interactions.
As such, devising new techniques to study cells in a more complex 3D environment is very useful.
Organoids resulting from cells grown in 3D culture allow for the study of cells in an environment that more closely resembles their natural counterpart. Such 3D structures can be used for understanding normal and disease development, drug screening, and also could potentially reduce the use of animals in research.
The pancreas is an important organ with acinar tissue that generates the digestive enzymes, the ducts that provide the plumbing to get the enzymes to the intestine, and the islets of Langerhans for the regulation of the blood glucose.
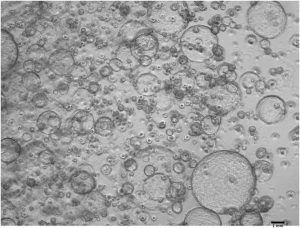
Isolated ductal cells robustly formed organoids after 10 to 14 days in Matrigel
The pancreatic duct cells are of interest as a potential source of pancreatic progenitor cells which can form new insulin-producing cells in adults, as well as being the origin of pancreatic cancer.
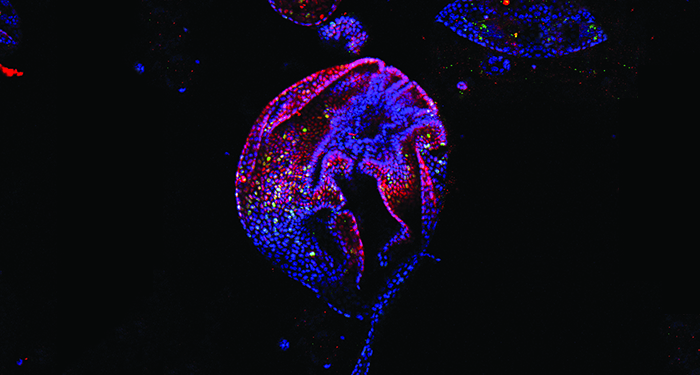
Recently Susan Bonner-Weir and her co-workers at the Joslin Diabetes Center, Harvard Medical School, devised a cost-effective protocol to robustly grow 3D organoids from mouse pancreatic ductal cells and developed an immunostaining protocol to stain intact whole 3D organoids without the need for sectioning.
The protocol details how to isolate pancreatic ductal cells and grow them as organoids.
This staining method will aid the study of cell–cell and cell–matrix interactions more precisely. Using this organoid model, they showed the diversity among the ductal cells and showed that pancreatic ductal cells maintain their ductal characteristics which makes these organoids an ideal model to study ductal cells in culture.