Inorganic/organic hybrid materials show great promise for use in photovoltaic devices, where the advantages of both types of materials could be combined to better harvest the sun’s energy. For the potential of hybrid materials to be fully realized, it isn’t sufficient simply to select the right materials, but the precise arrangement of these materials at small length scales can also be crucial to determining their ultimate properties and usefulness. Professor Chinedum Osuji and coworkers have developed a unique method for processing ZnO nanowires and polythiophenes that results in a hybrid structure with nanometer-scale ordering and a high crystallinity, showing great promise for the future of hybrid photovoltaics.
Inorganic/organic hybrid materials show great promise for use in photovoltaic devices, where the advantages of both types of materials could be combined to better harvest the sun’s energy. For the potential of hybrid materials to be fully realized, it isn’t sufficient simply to select the right materials, but the precise arrangement of these materials at small length scales can also be crucial to determining their ultimate properties and usefulness. Professor Chinedum Osuji and coworkers have developed a unique method for processing ZnO nanowires and polythiophenes that results in a hybrid structure with nanometer-scale ordering and a high crystallinity, showing great promise for the future of hybrid photovoltaics.
In order to understand why ordering is so crucial to the performance of hybrid photovoltaics, it is instructive to look closer at how such devices work. In its most basic form, the function of a photovoltaic device is to convert light energy from the sun into electrical energy (electrons and holes with negative and positive charges respectively) with a high efficiency. The light absorption usually occurs in the organic material, thereby creating an electron-hole pair. In order to extract useful work from the device, the electron must be transferred to the inorganic material, and then the electron and hole can be pulled to opposite sides of the device by the electric field in order to complete the electronic circuit. By ordering the materials at the nanoscale, it can be assured that the charge carriers have only a short distance to travel in order to complete this process. The molecular ordering within the polymer layer is very important where charge transport is much more favorable in a crystalline polymer than an amorphous one. Furthermore, the orientation is also key, where charge transport along polymer chains is more favorable than lateral hopping between chains. By controlling the arrangement of these materials as precisely as possible (and at multiple length scales), the losses from the device can be minimized to achieve a high power conversion efficiency.
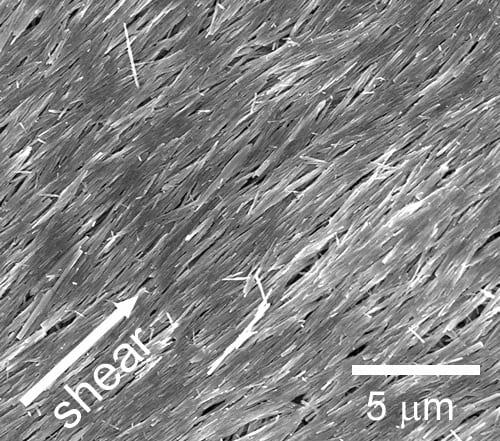
Achieving this type of complex ordering at small length scales is not easy. To arrange each of these domains by hand, we would require nano-sized tools, and a macro amount of patience. However, by taking advantage of intermolecular and other long-range forces we can coerce the materials to order themselves. This class of methods is fittingly called “self-assembly”. Professor Osuji and coworkers were able to take advantage of electrostatic forces to graft a thiophene-based polymer onto the surfaces of ZnO nanowires, creating a new crystalline phase that does not exist when the polymer is processed alone. Then, in order to arrange the coated nanowires, a shear force was applied to the hybrid material to achieve the ordering shown in the image above. As an alternative method, the authors also achieved very similar ordering by applying a magnetic field after a small amount of cobalt was incorporated into the nanowires. In both cases, the result was an ordered hybrid material boasting a near-ideal geometric arrangement for charge collection in photovoltaic devices. With creative methods such as these, the future of hybrid materials appears bright, and we may reap the rewards in the next generation of solar cells.