 Polymers produced by ring-opening metathesis polymerization (ROMP) generally have flexible backbones and relatively low glass transition temperatures (Tg). Higher Tg values, well above room temperature, would impart rigidity to the polymer and stiffness and structural integrity to parts formed from it. Moreover, monomers forming glassy polymers would be versatile components of block copolymers, as they could constitute the glassy “hard” blocks in thermoplastic elastomers. These thermoplastic elastomers are in great demand because they can be – in contrast to classical elastomers – processed in melt and the parts can be melted again. Ring-opened polymers of norbornene and substituted norbornenes represent a versatile class of materials.
Polymers produced by ring-opening metathesis polymerization (ROMP) generally have flexible backbones and relatively low glass transition temperatures (Tg). Higher Tg values, well above room temperature, would impart rigidity to the polymer and stiffness and structural integrity to parts formed from it. Moreover, monomers forming glassy polymers would be versatile components of block copolymers, as they could constitute the glassy “hard” blocks in thermoplastic elastomers. These thermoplastic elastomers are in great demand because they can be – in contrast to classical elastomers – processed in melt and the parts can be melted again. Ring-opened polymers of norbornene and substituted norbornenes represent a versatile class of materials.
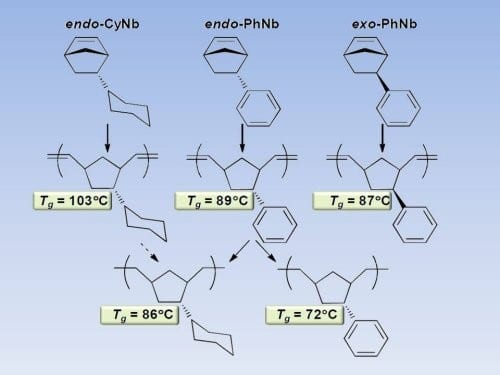
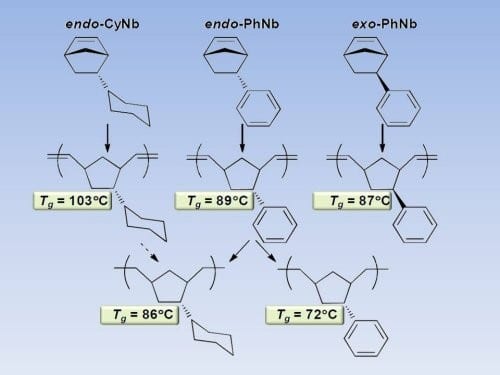
The group of Richard A. Register from the Princeton University have now showed that adding a phenyl side group to the norbornene ring can raise the Tg by some 50oC, to nearly 90oC. These polymers can be produced by “living” ROMP with well-defined Mo- and Ru-based catalysts, allowing for their easy incorporation into more complex polymer architectures. Surprisingly, the stereochemical configuration of the substituent (endo/exo) has a negligible effect. Nevertheless hydrogenation of the phenyl sidegroup to a cyclohexyl ring allows to further increase the Tg by 14oC to reach over 100oC. This also means that the polymer matches or even exceeds the Tg of polystyrene, a popular “hard” block in thermoplastic elastomers. This increase in Tg via side group saturation essentially compensates for the Tg decrease incurred when the backbone is concomitantly hydrogenated and its flexibility increased
These results further illuminate the direction and magnitude of changes in Tg which can be achieved in allhydrocarbon ROMP polynorbornene derivatives through simple and practical chemical modifications, either by changing the monomer structure or through post-polymerization hydrogenation.