The human immunodeficiency virus-1 (HIV-1)—a major health problem for humankind—accounts for around 95% of all infections worldwide.
HIV-1 destroys CD4+ lymphocytes (a type of white blood cell) and impairs cell-mediated immunity, therefore increasing the risk of certain infections and cancers. The risk of subsequent manifestations, e.g., the acquired immune deficiency syndrome (AIDS), is proportional to the level of CD4+ lymphocyte depletion.
Eradication of HIV-1 reservoirs or functional cures are very active research areas. Their success can lead to more effective treatment of HIV-1. The shock-and-kill approach has been well tested but has not effectively reduced the reservoir size in treated patients.
An article in Advanced Science by Thomas Lehner, Caijun Sun, Ling Chen, and co-workers addresses an important problem—how to target the latent viral reservoir which is responsible for long-term HIV-1 persistence and lack of a cure.
 Arsenic trioxide has been used to treat acute promyeolytic leukemia (APL) and hepatocellular carcinoma (HCC) patients in China.
Arsenic trioxide has been used to treat acute promyeolytic leukemia (APL) and hepatocellular carcinoma (HCC) patients in China.
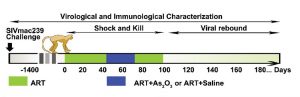
The team showed that arsenic trioxide can do four things: 1) specifically reactivate latent infection in both cell lines and primary CD4+ T cells from chronically infected monkeys and humans without activation of immune cells and inflammatory responses; 2) down-regulate expression of CD4 and CCR5 in T cells; 3) enhance T cell immune responses, and, more importantly; 4) fully suppress rebound of viral loads or delay the virological rebound.
What is noteworthy, the results were obtained in a well-validated macaque model which recapitulates HIV-1 pathogenesis and persistence during therapy in humans.
It is the first time that such compound is used with success and could represent a perfect candidate for a drug to help cure patients infected with HIV-1.
The specific activation of latent infection cells and the successful clinical use of arsenic trioxide makes it a very promising novel latency-reversing agent to achieve the goal of eradicating HIV-1.