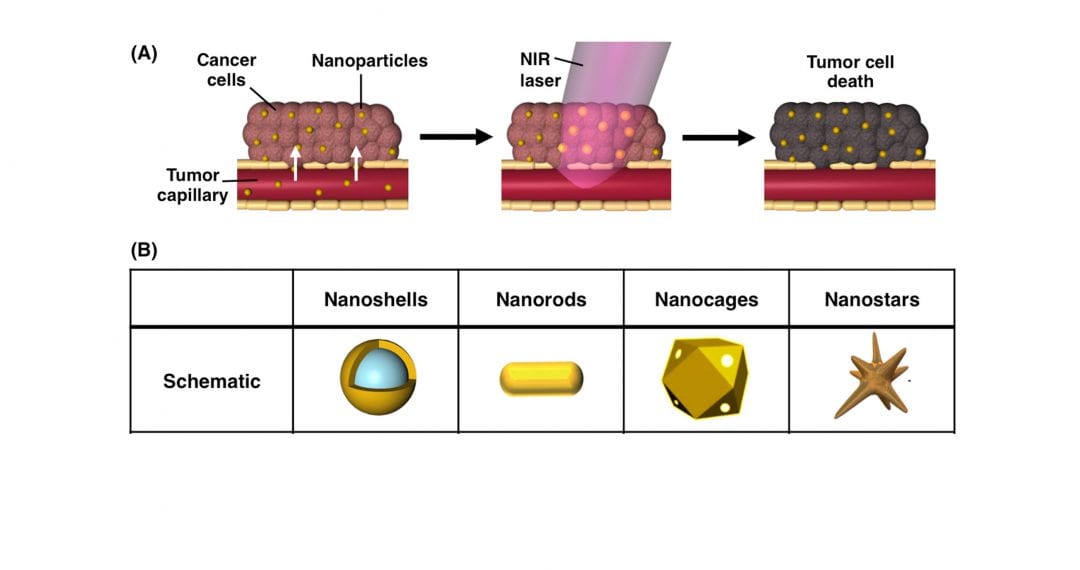
In the past decade, photothermal therapy has emerged as a promising treatment for solid tumors that offers high specificity and minimal adverse effects to healthy tissues. In photothermal therapy, nanoparticles embedded within tumors are exposed to tissue-penetrating near-infrared light, which causes the nanoparticles to produce enough heat to damage adjacent cancer cells. Photothermal therapy is most commonly mediated by gold-based nanoparticles, because they are biocompatible, and they enable simple surface functionalization with molecules that can extend circulation time and/or increase tumor penetration. Additionally, the structural dimensions of gold nanoparticles can be specifically designed to ensure that they maximally absorb near-infrared light and efficiently convert it into heat.
Impressively, researchers have extensively validated gold nanoparticle-mediated photothermal therapy as an independent treatment strategy in pre-clinical studies, and the technology is currently being evaluated in multiple clinical trials. More recently, researchers have begun investigating the use of photothermal therapy in combination with secondary treatment strategies, such as chemotherapy, gene regulation, and immunotherapy, to improve upon the overall therapeutic outcome.
In a recent review in WIREs Nanomedicine and Nanobiotechnology, Rachel S. Riley and Emily S. Day of the University of Delaware Department of Biomedical Engineering discuss photothermal therapy as both a standalone treatment strategy and as a method to enhance the anti-tumor effects of secondary therapies. While chemotherapies and other small molecule agents have dramatically improved patient outcomes, their success is limited by insufficient tumor delivery, off-target toxicity, and cellular resistance. As discussed by Riley and Day, researchers and clinicians can capitalize on the physiological and cellular changes that occur from photothermal therapy to overcome these limitations. For example, the heat generated by photothermal therapy can increase tumor vessel permeability for enhanced drug accumulation. In addition, the generated heat can work cooperatively with immunotherapies to eliminate both primary tumors and any disseminated metastatic disease.
These benefits, among others, can increase the success of both photothermal therapy and secondary treatments while reducing the required dosages of each. Overall, using photothermal therapy in combination with secondary treatments offers great potential for increasing tumor regression while minimizing off-target toxicity, and together these benefits should ultimately result in improved patient prognosis.
Contributed by the Authors