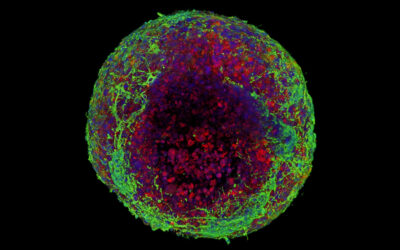
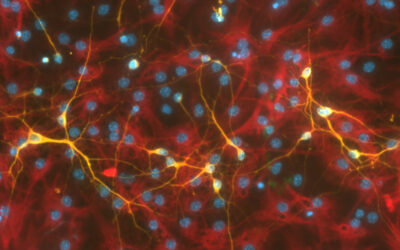
Researchers have demonstrated that the regeneration and reproductive capabilities of hydras, a relative of jellyfish, can be accelerated using heat-emitting gold nanoparticles. The precision and control offered by their approach is promising for targeting individual cells, a major goal of regenerative medicine.
High-intensity heat has been wielded as a weapon to kill cancer cells, and low-intensity red and blue light masks have become trendy tools for anti-aging and improving skin conditions like acne, but scientists have only more recently begun to explore the use of heat and light in regenerating tissue.
Gold nanoparticles generate localized heat
Claudia Tortiglione, a researcher at the National Research Council of Italy’s Institute of Applied Science and Intelligent Systems, and her team wondered if mild overheating in hydras could boost their regeneration process.
To do this, they used gold nanoparticles capable of generating heat when exposed to near-infrared light, which is invisible to the human eye.
“The gold nanoparticles, under infrared light stimulation, develop a tiny amount of heat inside the cells, which does not cause any damage or death, but might have beneficial effects, finally resulting in an enhanced efficiency of the regeneration process,” Tortiglione stated.
But the geometry of the particles matter. In their study, Tortiglione and her team used prism-shaped gold nanoparticles to tap into a phenomenon known as surface plasmon resonance.
“The gold nanoprisms […] exhibit surface plasmon resonance in the near-infrared region due to their triangular shape, and they can efficiently absorb light and convert this radiation into heat,” she explained.
Although prisms are ideal for generating heat, conventional spherical gold nanoparticles do not have the same properties, she pointed out.
The main advantage of using light as the trigger is the possibility to choose precisely where to deliver the heat. This is not possible when using an external heat source, which could potentially damage the tissue surrounding, for example, a wound.
Hydras as a model for tissue regeneration
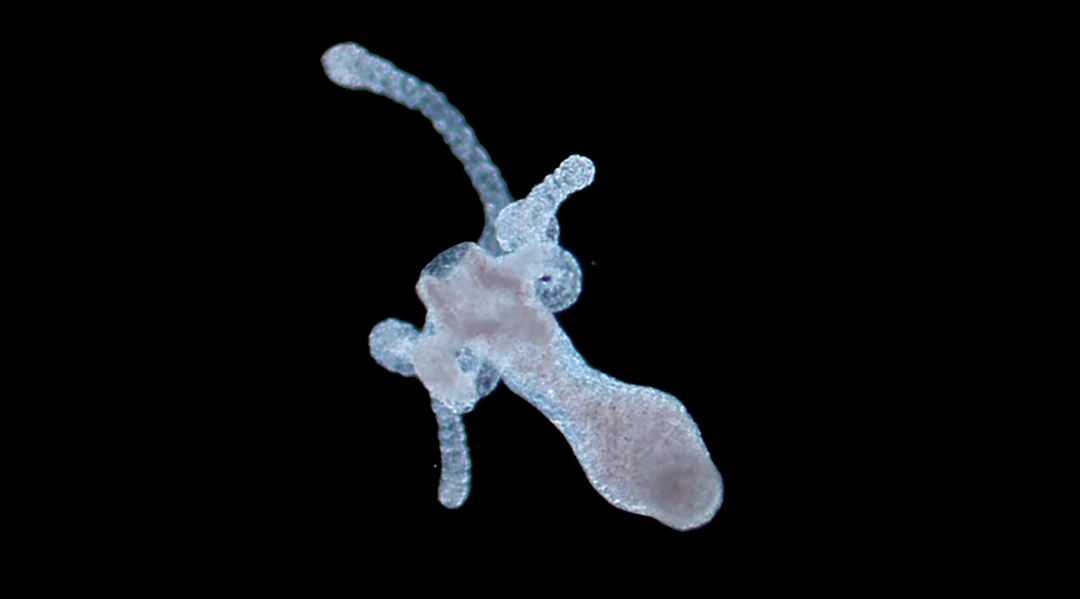
Hydras, a type of polyp that lives in freshwater, have a very simple anatomy that includes a soft, tube-shaped body and a hypostome, or mouth part, lined with tentacles. They have a “nerve net” in lieu of a brain and no muscles.
While certain organs in the human body regularly undergo self-repair, our regenerative capabilities are limited. Hydras, on the other hand, can perpetually regenerate their whole body. In fact, if a hydra is dissociated into single cells and the cells are recombined, a new hydra will grow. This superpower makes them an ideal model for studying tissue regeneration.
Tortiglione and her team were interested in testing whether hydras could regenerate an amputated head faster after absorbing the prism-shaped gold nanoparticles.
“[In a few hours], the nanoprisms were internalized efficiently in hydra tissue by simply soaking the polyps in a solution—basically freshwater—containing the nanoparticles,” she said.
Normally, when a hydra is decapitated, its epithelial cells come to the rescue, closing the wound within the first hour. Afterward, the hydra grows a new head, with the entire process taking around three days.
When the heads of hydras treated with gold nanoparticles were amputated, and the animals were illuminated with a near-infrared laser for five hours daily over two days, more hydras had advanced to the later stages of regeneration than those that were untreated.
Towards single-cell control
By counting the dividing cells, an increase in stem cells and epithelial cells was found in these polyps, which might explain the enhanced regeneration, said Tortiglione.
“The heat activates both the transcription of key genes involved in the stem-cell proliferation and in the heat stress response,” she elaborated. “These genes are normally activated following an injury or decapitation, but under [near-infrared light] stimulation, their activation was strongly anticipated.”
Using a thermal camera, the researchers found that the internal temperature of the nanoparticle-treated hydra increased and plateaued at around 7 °C after 20 minutes of near-infrared light exposure, which is sufficient to promote regeneration without damaging the tissue. A higher temperature might have induced cell death, according to Tortiglione.
The researchers also monitored budding, the asexual reproductive capability, of three nanoparticle-treated mother hydra. During the normal budding process, new hydras form on the side of a mother hydra’s body and then detach in about three days.
Each day for two weeks, the researchers fed the mother hydras and illuminated them with near-infrared light for five hours. They found that the hydras had a slightly higher reproduction rate than untreated hydra, producing a larger population of offspring. This enhanced reproduction rate is another effect of the enhanced epithelial cell proliferation.
In the future, the researchers aim to test the particles in pre-clinical skin regeneration models, as skin can be easily illuminated.
“We can imagine to treat a lesion with the gold nanoparticles and then switch on the light on the lesion to promote locally the wound healing without affecting the healthy surrounding tissues,” Tortiglione commented.
But targeting single cells is the ultimate goal. In all their experiments, the researchers illuminated the whole animal — not single cells — and observed the overall response.
“It would be interesting to focus the [near-infrared] laser on a single cell and observe the effects elicited by the heat,” she shared. “This is the core of a project we are working on at the moment.”
Reference: Natalia Dell’Aversano, et al. Optical Switchers to Manipulate Intracellular Pathways and Boost Tissue Regeneration, Advanced Functional Materials (2024). DOI: 10.1002/adfm.202405400