Researchers have given graphene a boost, synthesizing a new composite that combines its remarkable properties with a strong response to magnetic fields. This discovery holds promise for advancing spintronics, a branch of electronics where information transfer and storage relies not only on electrons’ movement but their magnetic properties.
“Ferromagnetism [magnetism] is important for [graphene’s] applications in electronic devices,” Qun Xu, a professor at Zhengzhou University in China and lead author of the study, explained in an email. “For example, in magnetic memory devices, ferromagnetic materials are commonly used to store and read information. The properties of ferromagnetic materials give magnetic memory devices the advantages of high density, low power consumption, and fast read/write speeds.”
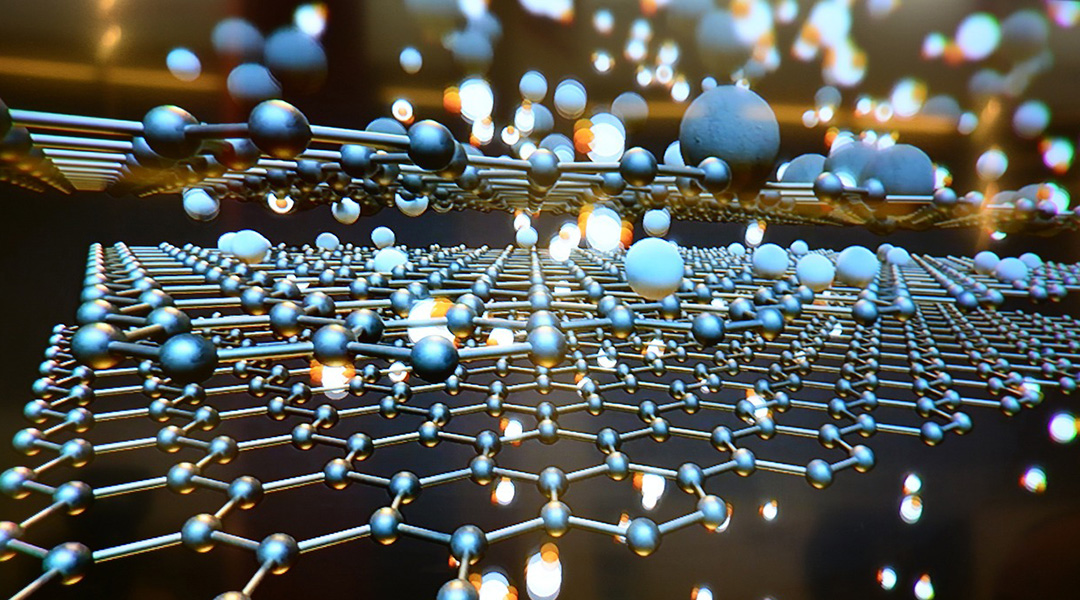
Graphene, a one-atom-thick layer of carbon, boasts exceptional strength, flexibility, and transparency, and has found application in various industries such as energy storage, sensors, and biomedicine.
“Graphene is a two-dimensional material consisting of a single layer of carbon atoms on which the surface electrons are free to move, so it has extremely high electrical conductivity,” said Xu.
As such, it has potential in advancing electronics and computer science. However, for widespread use it needs to exhibit ferromagnetism, meaning it must respond strongly to magnetic fields.
Making graphene ferromagnetic
To address graphene’s lack of ferromagnetism, Xu and his colleagues synthesized a new hybrid material, incorporating multiple layers of carbon atoms along with hydrogen and oxygen atoms.
They achieved this by subjecting graphene to carbon dioxide under pressures more than a hundred times higher than atmosphere, which not only forced it to bond with the graphene layers but also infused oxygen atoms into the crystalline structure. Hydrogen atoms came from hydrogen peroxide present as a byproduct during this process.
The magnetic properties of electrons depend greatly on how they interact with their atoms. By deforming the material’s crystal lattice or adding additional atoms its properties can be manipulated, and this is exactly what the authors achieved. Their modification of graphene’s structure led to the necessary changes in the properties of electrons, making them more susceptible to an applied magnetic field.
“We have synthesized amorphous graphene oxide with room-temperature ferromagnetism by introducing abundant oxygen-containing groups and defects into monolayer graphene, which are further self-assembled with the assistance of supercritical carbon dioxide,” Xu added.
Metal free
An essential component of this study, in contrast to conventional approaches, is the fact that the team did not have to rely on metals to induce magnetism in graphene, as their scarcity and environmental impact pose challenges to sustainability.
The team believes that the material they have obtained will find its way into electronics in the near future, accepting that there are also difficulties along the way that will need to be overcome.
“We’re doing our best to put it into practice, maybe in the next 3–5 years,” said Xu, hopeful. “Large scale production is the remaining hurdle.”
Beyond its technological implications, the study holds significance in the realm of carbon capture as it uses and traps carbon dioxide — a potent greenhouse gas. By repurposing CO2 in processes like this, researchers could potentially positively contribute to net zero targets.
“In the future, besides the fascinating room-temperature graphene oxide, we hope to use CO2 to obtain more interesting materials,” concluded Xu.
Reference: D. Zhang, B. Gao, S. Xu, C. Niu, and Q. Xu, Room-Temperature Macroscopic Ferromagnetism in Multilayered Graphene Oxide, Advanced Physics Research (2024). DOI: 10.1002/apxr.202300092
Feature image credit: seagul on Pixabay