Stem cells are a blank slate — these cells have not yet received instructions to develop into specific tissues. The instructions come via a complex series of events involving physical forces, hormones, and even electricity, all of which guide the handful of cells in an embryo to transform into all the bone, skin, lungs, and tissue of a living organism.
Medicine has long been interested in harnessing these cells for their regenerative capabilities, but the challenge has always been untangling the right combinations of factors that steer a stem cell toward a desired fate. This is especially tricky for neurons, which make up the central nervous system, as they are notorious for their inability to heal or regenerate.
Researchers previously found that stem cells can be coaxed to produce neurons if supplied with an electrical charge. This raises a few important questions such as how can both the stem cells and the electrical current be delivered to a patient in a minimally invasive way?
Wired electrodes and batteries are not ideal as they are not biocompatible, and patients must endure multiple surgeries to have these implanted and removed. It’s also unclear as to why an electrical current encourages stem cells to become neurons and how can labs best study it?
For Hamideh Khanbareh, a professor of mechanical engineering at the University of Bath, the answers lie in a fascinating type of material that safely produces an electrical current when placed under mechanical stress.
Bend it, move it, charge it!
Piezoelectric materials are unique in that they produce an electrical charge in response to a mechanical force. Bending them, moving them, pressing down on them all produces a charge. This seems ready made to provide the charge for stem cell growth in the body.
But some of the most efficient piezoelectric materials are crystals or lead ceramics, both of which are not compatible with living tissues. Khanbareh therefore turned to polymer-based materials to balance piezoelectric ability with biocompatibility.
“The idea behind introducing the polymer was matching the mechanical properties of the piezoelectric material with that of the tissue because that wouldn’t be possible in a ceramic based system,” she explained. Polymers are flexible gel like materials that are made of repeating units of organic molecules, making them suitable for interacting with soft living tissues.
However, piezoelectric polymers are rather limited and difficult to work with. “There’s not much engineering you can do in these systems,” said Khanbareh. To get the best properties from both ceramics and polymers a hybrid was needed.
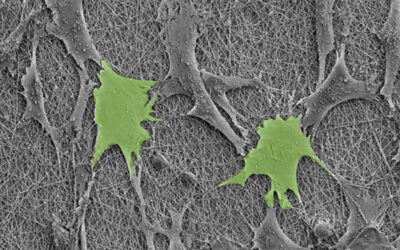
The hybrid design took micron-sized bits of piezoelectric ceramics and placed then within a polymer matrix. Then, in a process called dielectrophoresis, an electrical field is applied to the material, causing the ceramic bits to align in chains.
Now when a force is applied, the chain distorts and transmits the charge. In this way, the ceramic-polymer mix is both biocompatible and able to efficiently produce a current for the developing cells.
Growing neurons
The task of testing whether this material efficiently encourages stem cells to become neuronswas given to Vlad Jarkov, then a Ph.D. student in the Khanbareh lab.
Aside from optimizing the production of the ceramic-polymer composite and designing a set-up to accommodate the cells, he needed a crash course in neuroscience. “The stem cell stuff was new to me when I started,” said Jarkov. “Learning cell culture in an actual neuroscience lab was difficult.”
With the help of collaborators Chris Adams and Imaan Waqaar at Keele University, they took stem cells directly from mouse embryos, grew them for a few days, and placed them on the ceramic-polymers to and tracked how quickly and efficiently the cells developed into neurons.
This delicate process was necessary, as he explained, because using primary cells directly from mouse embryos is the best way to mimic stem cells that will eventually come from humans.
“If we gave them a few cycles to differentiate, we might start losing some of their stemness, and their ability to differentiate into the into the cell lines that we want,” he said. “So, that was a big challenge.”
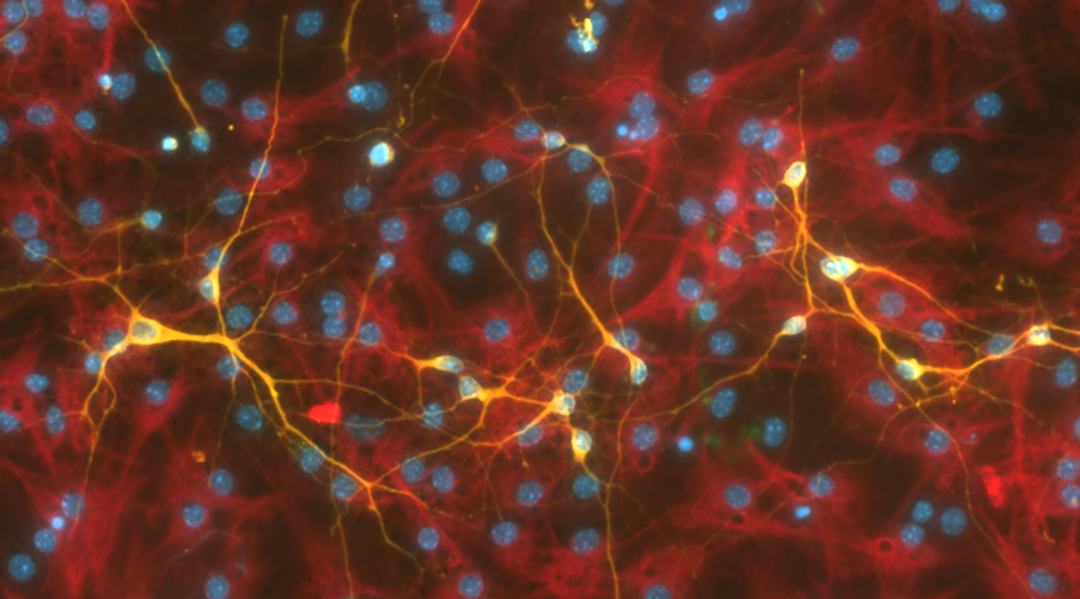
With the cells in hand, he was able to compare whether the piezoelectric surface produced more neurons. Compared to controls where the ceramic-polymer was not activated or the cells were on glass coverslips alone, more differentiated neurons were produced on the charged piezoelectric ceramic-polymers.
This demonstrates the feasibility of the material for medical researchers and neuroscientists who are developing treatments or studying how stem cells operate.
According to Jarkov, the material is now available for others to experiment with. “We made an interesting material with fantastic properties. It’s tunable. It’s scalable. It shows increased neuron differentiation from neural stem cells,” he said. “All of these things are great, but to really take it to a place that’s useful for society we need neuroscientists and the full range of people to interact with this work and get involved.”
Reference: Vlad Jarkov, et al., Enhancing Neural Stem Cell Stimulation with Structured Piezoelectric Composites: An In-Vitro Study, Advanced Engineering Materials (2023). DOI: 10.1002/adem.202300696