Illustration by Daniela Guzmán Angel
When teaching materials chemistry, I always ask my students to explain in a few sentences how certain materials have changed our world. Some of my favorites include the silica in fiber optic communications, silicon in microelectronics, gallium nitride in LEDs, and lithium cobalt oxide in batteries, to name a few. Though it may be tempting to envision these materials as the product of precision engineering and targeted design, many of these groundbreaking materials find their origins as rediscovered gems buried deep within the dusty archives.
Following this, a recent report had inspired me to include barite, or BaSO4, to my list of ancient but revolutionary materials. Traditionally used in barium meal, a radio-opaque suspension consumed before X-ray or CT scans of digestive tracts, barite holds the potential to decrease urban climate change contributions by presenting a non-powered alternative to air conditioning: passive radiative cooling.
In comparison, most of the cooling technologies used in the plethora of modern applications are based on Joule-Thompson compression-expansion systems, which are notorious for their use of greenhouse gas coolants, energy costs, and CO2 emissions. The effects of these costly systems will only increase with rising populations and global warming as the number of air conditioning systems are projected to increase from 1.2 billion in present day to 4.5 billion by 2050. Much of the growth will occur in cities where temperature increases are more pronounced due to the urban heat island effect.
Without intervention, the expansion of current cooling technology is expected to contribute an additional 167 gigatons of CO2 emissions by 2050, consume a major fraction of the global energy supply, and cause 0.5°C global warming by 2100, driving a vicious cycle of increased emissions, rising temperatures, and greater cooling demands.
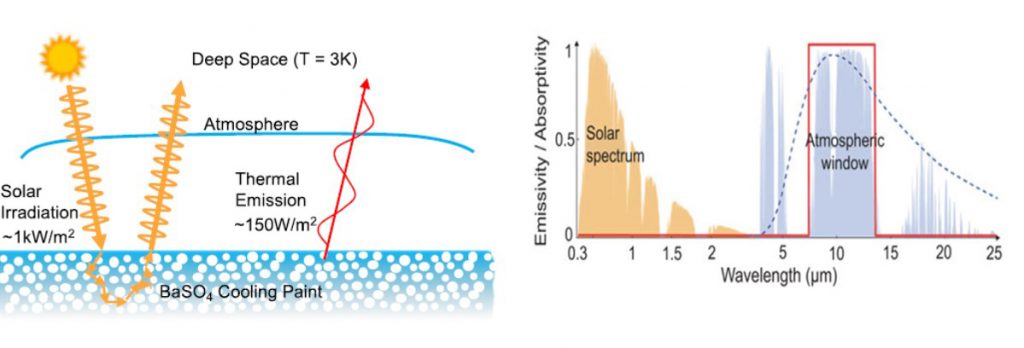
Now this is where barite comes into the story as the spearhead for a new generation of efficient passive daytime radiative cooling (PDRC) materials. These materials are efficient because of their optical properties — low absorptivity and high reflectivity in the solar spectral wavelength range minimizes heat uptake, and high emissivity in the wavelengths best able to penetrate the “atmospheric window” maximizes cooling potential.
Key materials performance metrics that make barite an ideal choice include its deep ultraviolet electronic bandgap for low solar absorptance and heating, and 9 micrometre phonon frequency for high emissivity in the sky window for high cooling. The particle size distribution can be tailored to optimize its reflectivity and emissivity, and average cooling power at 97.6%, 0.96, and 117 W/m2, within striking distance of the maximum possible 100%, 1, and 150 W/m2, respectively. The titleholder 60 vol% BaSO4/acrylic has a reflectivity of 98.1% and emissivity of 0.95, making it a candidate for a PDRC paint.
Under ideal conditions with dry atmosphere, the maximum achievable radiative cooling power of barite at 20-30°C is an impressive 150 W/m2 and 100W/m2 at 0°C, with the former delivering a temperature reduction in the range of 4-10°C.
Despite these encouraging values, the material has an Achille’s heel: water. Cooling rates are impeded under moist atmosphere due to a reduction in transmission at water absorption bands. For example, at humidity levels above 60%, the cooling power was found to drop below 50 W/m2.
It turns out that the PDRC properties of barite particle films and particle-polymer paints come close to achieving the ideal absorption, reflection, and emission effects, making it a serious contender for radiative cooling of a range of surfaces to sub-ambient temperatures.
Despite barite’s promising outlook, this does not mean that wide-spread implementation should be initiated without comprehensive economic and environmental analysis of PDRC coating benefits on an application-by-application basis. This involves evaluation of net saving in energy and coating costs and overall reduction in the CO2 footprint attributed to replacing traditional cooling methods, which vary by geography, pollution levels, and local climate. For example, the implementation of barite has been rigorously proven to be more efficient than PDRC, with CO2 emission reduction and decreased energy expenditure on cooling when applied as a coating to rooftops compared to white painted and conventional roofs.
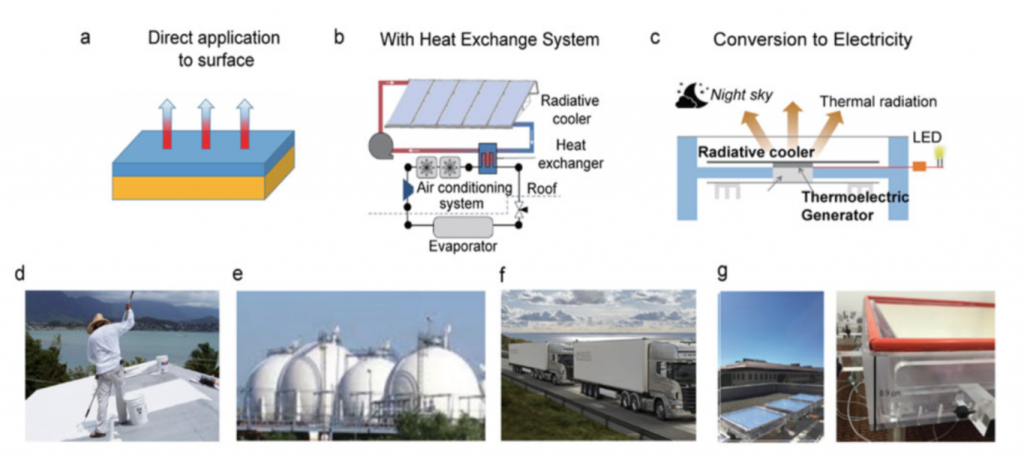
Again and again, I am both awed and inspired by the ingenuity in the scientific community. While the trend in modern chemistry is to employ flashy machine learning and automation to expedite the discovery of novel materials, let us not forget that sometimes our solutions lie right underneath our feet in the hidden gems of our scientific history.
Written by: Geoffrey Ozin, Joel Loh, and Jessica Ye
Solar Fuels Group, University of Toronto Ontario, Canada, Email: [email protected], Web sites: www.nanowizard.info, www.solarfuels.utoronto.ca, www.artnanoinnovations.com

















