 Large-scale energy storage is a key strategy to effectively utilize intermittent renewable energy. The electrochemical storage of energy using rechargeable batteries plays an important role in this context. Despite the many types of rechargeable batteries that have been investigated, few can meet the requirements of capacity, stability, cost, and safety simultaneously.
Large-scale energy storage is a key strategy to effectively utilize intermittent renewable energy. The electrochemical storage of energy using rechargeable batteries plays an important role in this context. Despite the many types of rechargeable batteries that have been investigated, few can meet the requirements of capacity, stability, cost, and safety simultaneously.
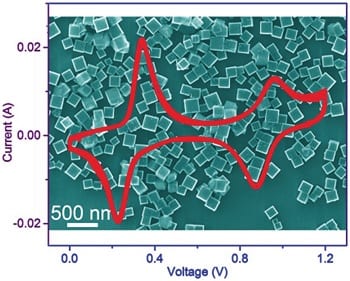
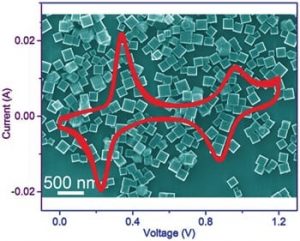
Researchers have reported the synthesis of potassium iron (II) hexacyanoferrate dehydrate nanocubes with a high potassium ion content and their application as an electrode material in aqueous electrolyte potassium-ion batteries. The high potassium ion content ensures that the new electrode material can function as an ample potassium ion reservoir in the aqueous potassium-ion battery. A discharge capacity of 120 mA h g-1 (the highest value for an aqueous potassium-ion battery to date) was obtained with > 85 % capacity retention over 500 cycles at a rate to fully charge or discharge the batteries in three minutes. The high capacity can be ascribed to the high potassium ion content together with the two accessible one-electron redox processes. An open-framework structure enables structural integrity and facilitates the fast kinetics. Furthermore, all of the elemental components, in particular iron and potassium, are earth-abundant and non-toxic. These properties, together with the high capacity, outstanding cycle life, safety, and low-cost of active materials make that aqueous potassium-ion batteries can become the most attractive battery system for large-scale energy storage applications.