 In general, alloys consist of more than one element with one of the elements being the primary element. For example, the primary element in steel is Fe with a minor amount of C and other alloying elements. Material engineers and scientists consider the primary element as a base metal, matrix, or solvent, and the minor elements are considered as a solute. For many decades, material engineers and scientists have developed new alloys based on one primary element concept. They widely believe that multi-principal elemental alloys would have poor mechanical properties due to the anticipation of compound formation which may have negative effects on the mechanical property.
In general, alloys consist of more than one element with one of the elements being the primary element. For example, the primary element in steel is Fe with a minor amount of C and other alloying elements. Material engineers and scientists consider the primary element as a base metal, matrix, or solvent, and the minor elements are considered as a solute. For many decades, material engineers and scientists have developed new alloys based on one primary element concept. They widely believe that multi-principal elemental alloys would have poor mechanical properties due to the anticipation of compound formation which may have negative effects on the mechanical property.
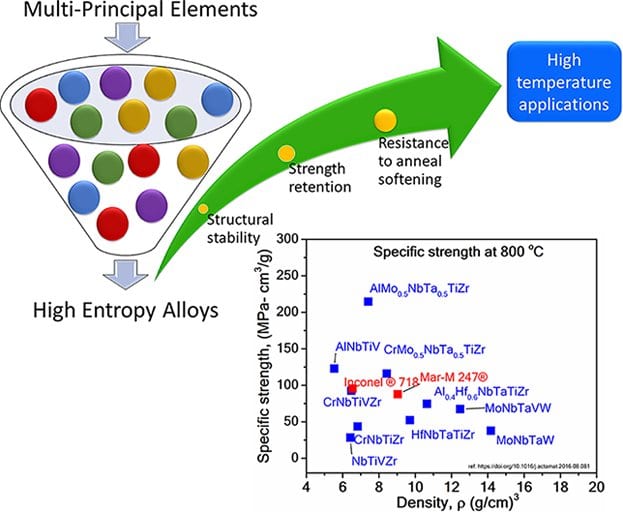
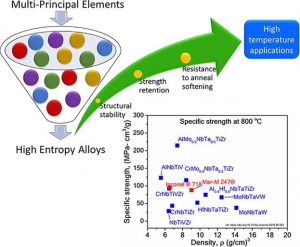
In the past few years, alloys with multi-principle elements known as high entropy alloys (HEAs) have gained wide attention due to the formation of simple structure and unique properties. The sluggish diffusion, high fracture toughness at cryogenic temperature, and strength retention at higher temperature are few of the unique properties reported for HEAs.
In a review in Advanced Engineering Materials, Sathiyamoorthi Praveen and Hyoung Seop Kim systematically summarize advancements in the field of HEAs as a potential emerging material for high-temperature applications. The microstructural evolution, resistance to anneal softening, phase stability, age hardening behavior, oxidation resistance, and high-temperature mechanical properties of various HEAs is summarized. The need for the profound investigation of the high-temperature behavior of HEAs based on creep experiments and the development of two-phase HEAs as a potential candidate for high-temperature applications is highlighted. The future challenge lies in the fundamental understanding of remarkable high-temperature properties of HEAs and its practical application for extreme environments.