Increasing the efficiency and stability of electrodes in lithium ion batteries is of huge importance for a myriad of energy applications. To this end, various strategies have been explored regarding improving the performance of battery electrodes, to prevent degradation and increase lifetime. One such strategy is the coating of the electrode by a protective material, often by an atomic layer deposition (ALD) method. ALD often requires high temperatures for successful deposition, which can present issues with full-formed electrodes if the binder material cannot withstand the deposition temperature. This limits the application of ALD coatings; however, provided the coating material is electrochemically active, the coating can be deposited instead on the electrode precursor powder. The powdered electrode material is then mixed with a binder and conductive carbon material and formed into an electrode. Good electrochemical activity of the coating material is necessary, as if the material does not have good electron and lithium conduction properties then there will be little to no contact between the electrode material and the conductive carbon.
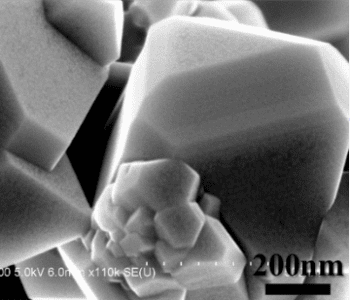
 Researchers in Canada have demonstrated an electrochemically active coating for use with LiNi0.5Mn1.5O4 (LNMO) cathode electrodes, by coating powdered LNMO electrode with several layers of the electrochemically active FePO4. The amorphous FePO4 coating forms an ultrathin layer around the powder, approximately 2 nm thick. To determine the optimal amount of coating for the best electrochemical properties, as required for cathode operation, several coated powder samples were prepared at varying deposition times of 5, 10, 20, and 40 ALD cycles.
Researchers in Canada have demonstrated an electrochemically active coating for use with LiNi0.5Mn1.5O4 (LNMO) cathode electrodes, by coating powdered LNMO electrode with several layers of the electrochemically active FePO4. The amorphous FePO4 coating forms an ultrathin layer around the powder, approximately 2 nm thick. To determine the optimal amount of coating for the best electrochemical properties, as required for cathode operation, several coated powder samples were prepared at varying deposition times of 5, 10, 20, and 40 ALD cycles.
Electrodes formed from this coated LNMO powder have shown excellent performance as cathode materials, with high capacity retention and Coulombic efficiency. The capacity retention of the electrode increases with increased amount of deposited FePO4, with a LNMO-40 showing a capacity retention of approximately 100% after 100 charge/discharge cycles. On the other hand, the initial capacity of the electrode is reduced by the coating, though the capacity retention of the bare LNMO electrode is only 80% after 100 cycles. The LNMO-10 electrode achieved the best balance of initial capacity, capacity retention, and Coulombic efficiency, with excellent performance even at high current densities.
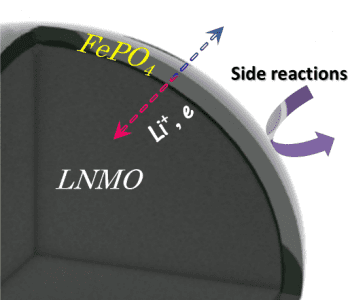
The increase in capacity retention and Coulombic efficiency of the coated electrodes is due the coating acting as a diffusion layer and electrochemical buffer. Reaction between any electrode and the electrolyte lead to the formation of a solid–electrolyte interface on the surface of the electrode. This layer has high resistance, and impedes the performance of the electrode. In this case, the FePO4 coating protects against the formation of this layer, and its thickness is reduced compared to the bare LNMO electrode. In addition, the electrolyte reduces Mn4+ ions in the electrode to Mn2+. Not only does this oxidize and degrade the electrolye, but the Mn2+ ions dissolve in the electrolyte and are deposited as Mn metal at the anode, harming the performance of both the cathode and the anode. The electrochemically active coating inhibits the direct contact of the electrode material and the electrolyte, while still allowing for good conduction of Li ions and electrons, since during cycling the FePO4 layer rapidly takes up Li, allowing for fast Li diffusion.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.