 Storing energy obtained from renewable energy resources is a challenge, since their dependence on natural conditions (e.g., sunlight, wind, tide, etc.) makes their availability intermittent. There has, therefore, been a need to develop low-cost, environmentally benign and sophisticated energy-storage devices such as supercapacitors and batteries.
Storing energy obtained from renewable energy resources is a challenge, since their dependence on natural conditions (e.g., sunlight, wind, tide, etc.) makes their availability intermittent. There has, therefore, been a need to develop low-cost, environmentally benign and sophisticated energy-storage devices such as supercapacitors and batteries.
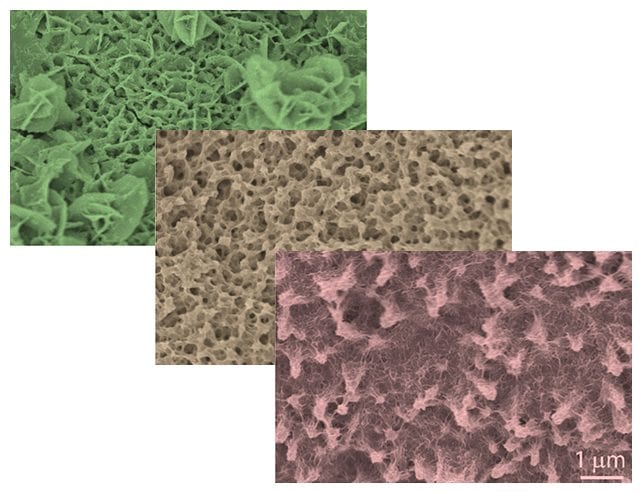
The performance of supercapacitors mostly depends on the electrode materials. Nickel sulfide, Ni3S2, an abundant and cheap material, has a high theoretical specific capacitance and good conductivity, which are two important characteristics of ideal supercapacitor materials. To take the full advantage of its material properties, however, the structure of Ni3S2 should be designed to allow efficient electrolyte diffusion and electron transport.
In recently published work in ChemNanoMat, Gamze Yilmaz and Xianmao Lu at the National University of Singapore have successfully prepared a 3D Ni3S2 network with tailored structure and composition. By introducing sulfur ions under hydrothermal reaction conditions, hierarchical porous structures of Ni3S2 were grown on nickel foam that acted both as a backbone and nickel source. With the 3D porous Ni3S2 as the positive electrode and activated carbon as the negative electrode, asymmetric Ni3S2//AC supercapacitors were built and exhibited excellent rate capability (72.5 % of the initial capacitance retained when the current density increased from 6 to 40 mA cm-2), good cycling stability (92.7 % retention of the initial capacitance after 5000 cycles) and high energy density (1.54 mWh cm-3 at a power density of 0.07 W cm-3).
The excellent performance of the device can be attributed to the following features: 1) nickel foam behaves both as an excellent conducting backbone and as the source material for the growth of nanoporous Ni3S2; 2) the porous nickel backbone provides excellent mechanical stability and allows easy access of electrolyte ions to electroactive surfaces via short pathways, 3) the binder-free structure offers intimate contact between the active Ni3S2 material and the conductive substrate facilitating charge transfer.
This article is part of a special issue on Nanomaterials for Energy Conversion and Storage in ChemNanoMat ((link to: www.chemnanomat.org)). Click here ((link to: http://onlinelibrary.wiley.com/doi/10.1002/cnma.v2.7/issuetoc)) to see the other articles in the issue.