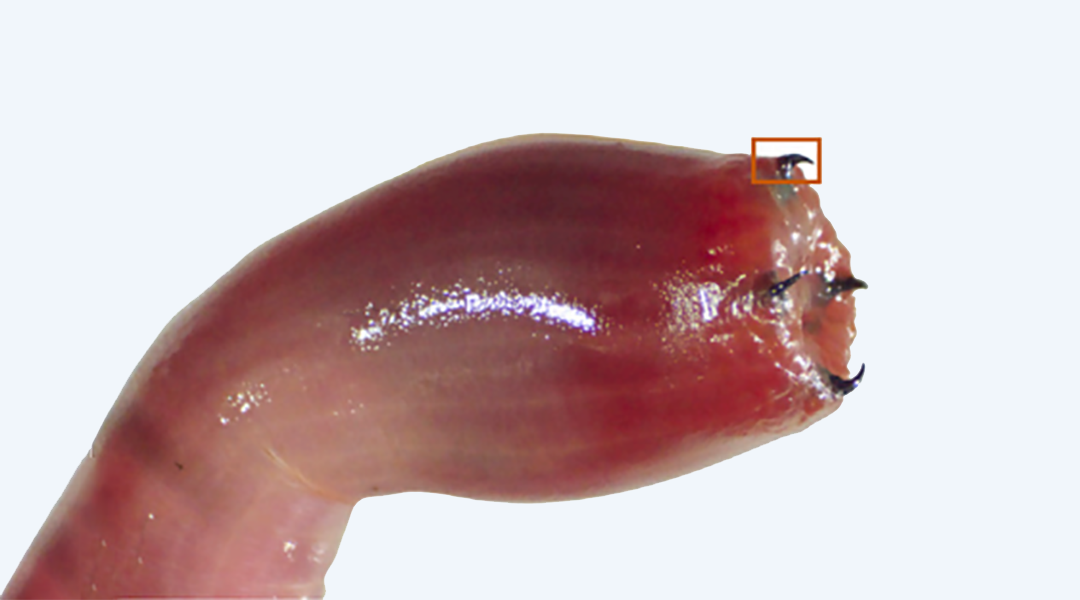
Made of protein, melanin, and a surprising amount of copper, the sharp, metallic jaws of a bloodworm help fight and fend off its enemies. While scientists have long known the composition of the bloodworm’s jagged jaws, how they were made was previously undiscovered.
Now, scientists have illuminated the process of how bloodworms’ copper teeth are formed, and found that a single protein works in multiple ways to build some of the strongest little jaws in the animal kingdom.
“Over billions of years, nature has evolved many ingenious solutions to problems that stump chemists and engineers,” said Matthew Harrington, a researcher at McGill University, Canada who was not involved in the study, in an email.
In the study published in the journal Matter, researchers identified a protein found in the coppery teeth of bloodworms capable of a variety of functions that may help inspire synthetic materials.
Bloodworms’ copper teeth
A type of segmented worm, bloodworms burrow into the mud in shallow coastal waters. To a bloodworm’s mouthparts come attached four black jaws that both grip and shoot venom. Around 2 mm in length, the worm’s jaws are a composite of protein, melanin, as well as mineral and ionic copper.
“Jaws are durable load bearing ‘tools,’ like our teeth in many respects, but unlike teeth, they are not made within the confines of living tissue,” explained Herbert Waite, a researcher at the University of California, Santa Barbara and one of the study’s corresponding authors. “Rather, they are made at the interface between living tissue and seawater.”
Waite and his team were able to test the behavior and properties of the bloodworm’s jaw protein using an identical, lab-made protein. For a protein largely made up of only two kinds of amino acids, the jaw protein was found to carry out several different functions — an unusual range of multitasking for a relatively simple molecule. Waite’s team termed their find the multi-tasking protein or MTP.
“The dual catalytic and load-bearing roles of the protein are not common in biology, and the simplicity of the protein sequence may lead to development of novel bio-inspired materials,” added Harrington.
In laboratory experiments, the protein binds copper and the resulting complex recoils from water in the same way oil and water repel one another. Next, the protein-copper compound triggers the conversion of a precursor of melanin — an amino acid called 3,4-dihydroxyphenylalanine or DOPA. Ultimately, the entire complex of protein, copper, and melanin assemble into films and fibers of a rigid nature. In this way, the jaw protein acts as an organizer and a manufacturer all at once.
Since all the experiments were performed outside the bloodworm, whether natural jaw formation occurs in the same way remains to be studied. “It is difficult to know exactly how these proteins might function in vivo since all experiments in the current study were performed in vitro on recombinant proteins,” said Harrington, who completed his doctoral research in Waite’s lab over a decade ago. “Nonetheless, the authors provide a very compelling story of the various roles that this protein might perform, and the demonstration of these behaviors is still of great value for bio-inspiration.”
Circumstances permitting, Waite would like to study the natural precursor of melanin in the bloodworm. “As in the novel Dune, nonhuman organisms should not be written off as worthless or useless just because they are primitive or inconspicuous,” said Waite. “Emerging from the jaws of a bloodworm is a concept of a protein or polymer that directs all aspects of its own assembly into a useful complex material; this is technologically irresistible.”
Reference: William R. Wonderly, et al., A multi-tasking polypeptide from bloodworm jaws: Catalyst, template, and copolymer in film formation, Matter (2022). DOI: 10.1016/j.matt.2022.04.001
Feature image: Image of the everted proboscis of Glycera dibranchiata with its four jaws exposed, Right: Scanning electron microscope image of a Glycera jaw (Scale bar, 0.5 mm). Credit: Matter/Wonderly et. al.