 Physicists from the University of York have carried out new research into how the heating effect of an experimental cancer treatment works.
Physicists from the University of York have carried out new research into how the heating effect of an experimental cancer treatment works.
Magnetic hyperthermia is viewed as an attractive approach for the treatment of certain cancers as it has no known side effects compared to more conventional therapies such as chemotherapy. It is particularly suitable for the treatment of prostate cancer and brain tumours. However, until now there has been no clear theoretical understanding of how it actually works.
Treatment by magnetic hyperthermia involves injecting magnetic nanoparticles directly into a tumour then placing the patient in a machine which produces an alternating magnetic field. The nanoparticles oscillate and heat is produced inside the tumour tissue. When the temperature rises above 42ºC cells begin to die. This heating process has been demonstrated to reduce tumour size.
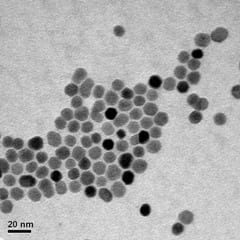
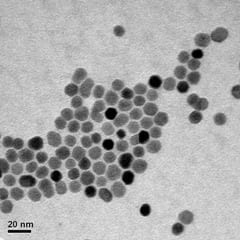
The study, by researchers from the University of York’s Department of Physics and Liquids Research Ltd, of Bangor, North Wales, showed that the amount of heat generated by magnetic nanoparticles can be understood when both the physical and hydrodynamic size distributions for the samples are known to high accuracy.
Lead author Dr Gonzalo Vallejo-Fernandez, from York’s Department of Physics, said: “While clinical trials have shown the potential of magnetic nanoparticles for cancer treatment, the mechanisms by which the heat is generated have not been fully understood. This understanding is critical to produce particles with optimised properties for specific applications at minimal dose.”
Previously the heat generated was impossible to predict as several mechanisms were involved. The new work has identified and quantified the mechanisms so that work can now begin to determine the dosage required for effective treatment.
Dr Vallejo-Fernandez said: “Through our study we have produced the first comprehensive assessment of how the heating effect in magnetic hyperthermia works. We are now in a position where we can do further work to calculate accurately the dose of magnetic nanoparticles and length of treatment required.”
For the study, the researchers used magnetic nanoparticles produced by a new technique by Liquids Research Ltd, which was developed under the EU project MULTIFUN (Multifunctional Nanotechnology for Selective Detection and Treatment of Cancer). The nanoparticles are very uniform in size and well separated, which enabled detailed experiments to be performed which broadly confirmed the accuracy of the calculations.
Dr Vijay Patel, Director of Liquids Research Ltd, said: “The development of this new theory coincided with our work on the new process to fabricate the nanoparticles enabling us to ‘design’ almost ideal particles for this application.”
Source: University of York