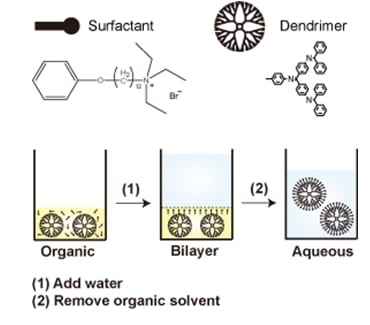
The researchers developed a new method to solubilize hydrophobic dendrimers using specific surfactant molecules.
Dendrimers have been attracting great attention as templates and stabilizing materials for finely size-controlled metal nanoparticles. Phenylazomethine dendrimers can be used to obtain precious metal, metal oxide, or bimetallic clusters with a perfect control of the number of atoms comprising one cluster molecule. Although they are quite stable in organic solvents, it is currently difficult to transfer them into aqueous media.
K. Yamamoto and co-workers (University of Tokyo) now show that a specific surfactant bearing an aromatic structure at the hydrophobic terminal allows solubilizing the pi-conjugating phenylazomethine dendrimers in water.
Under dilute condition, they form discrete micelles with a size close to that of the dendrimer. Condensation results in their aggregation; however, they remain in a homogeneous solution without precipitate formation. Furthermore, each micelle is well separated from the others. The excited state of the zinc porphyrin core in the dendrimer is significantly stabilized, which was not quenched at all.
This strategy will be of significant advantage not only for the solubilization in the aqueous phase but also stabilization of labile chemical species such as metal nano-clusters.