Small interfering RNAs (siRNAs) are produced by the cleavage and processing of double-stranded RNA and play a role in post-transcriptional gene silencing. They bind to complementary sequences in mRNA and bring about their cleavage. The selective degradation of the complementary mRNA specifically inhibits gene and protein expression. Unfortunately, small interfering RNAs can easily be degraded and show low permeability through the cell plasma membrane.
Protein nanocages exhibit many appealing properties making them attractive for delivery applications in general. They are robust, biocompatible, monodisperse, biodegradable and can be tuned by genetic engineering. Up to now, most research on protein nanocages for siRNA delivery has been based on virus-protein based nanocages. However, the use of nonviral protein nanocages would be advantageous. They do not face the safety concerns which limit the applicability of virus-protein based nanocages. Virus proteins are suspected of being responsible for gene mutation and carcinogenesis.
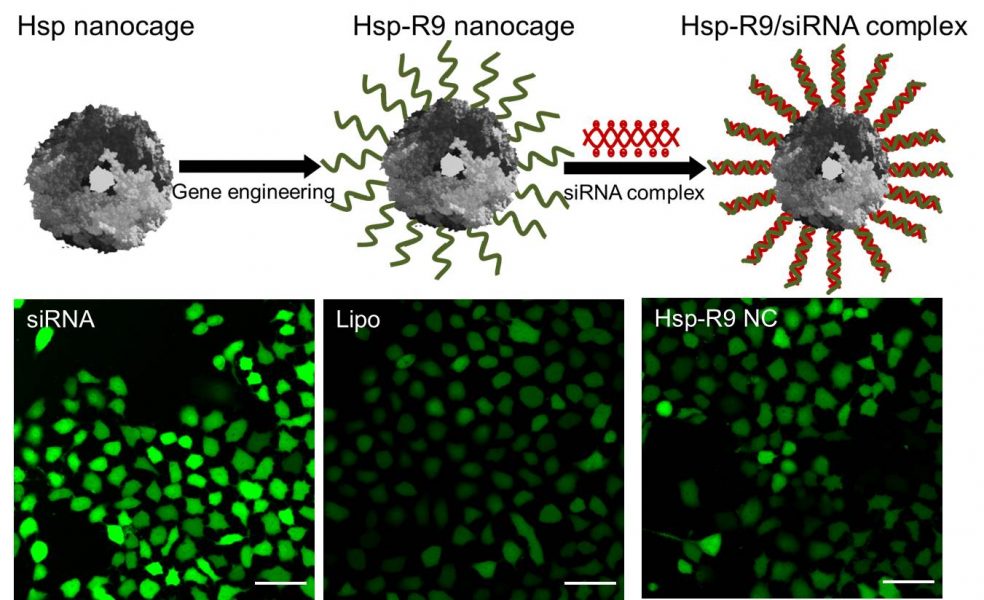
In their study, researchers from Beihua University in Jilin, China have designed a novel siRNA delivery system based on a heat shock protein (Hsp) nanocage. They introduce polyarginine peptides (R9) at the C-terminus of the heat shock protein via genetic engineering. siRNA can successfully be complexed to the Hsp-R9 nanocages. The condensed siRNA is stable against RNase degradation despite its location on the cage surface. The complexes show good cellular uptake and endosomal escape properties in tumor cells. Finally, their efficacy for gene silencing is investigated. The expression of EGFP can significantly be downregulated in EGFP-expressing HeLa cells. As the cavity of the nanocages could be loaded with drugs or imaging agents, the Hsp nanocages might be a powerful platform for the treatment of gene-related diseases or tumors.