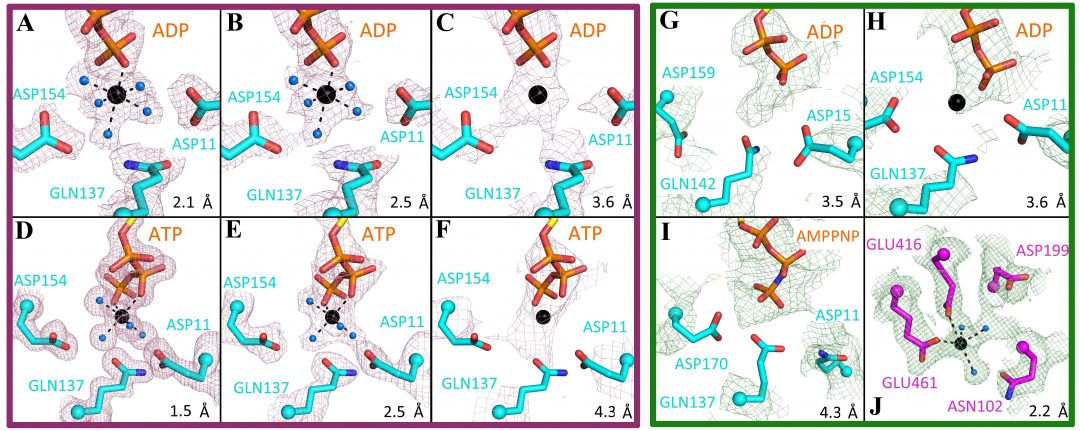
The development and improvement of various techniques such as X-ray free electron lasers, cryo-electron microscopy, micro electron diffraction and atomic force microscopy has led to a better understanding of various biological structures. However, biological filament systems are still underrepresented with regards to atomic resolution structures.
In their review in BioEssays, David Popp and co-workers first discuss the use of high resolution techniques in general terms and then take a closer look at their applications for biological filament systems. The studies recently performed in filament systems can be broadly classified into the following three categories: normal single particle reconstruction, isolated extended filaments and filament systems that are saturated with a binding partner.
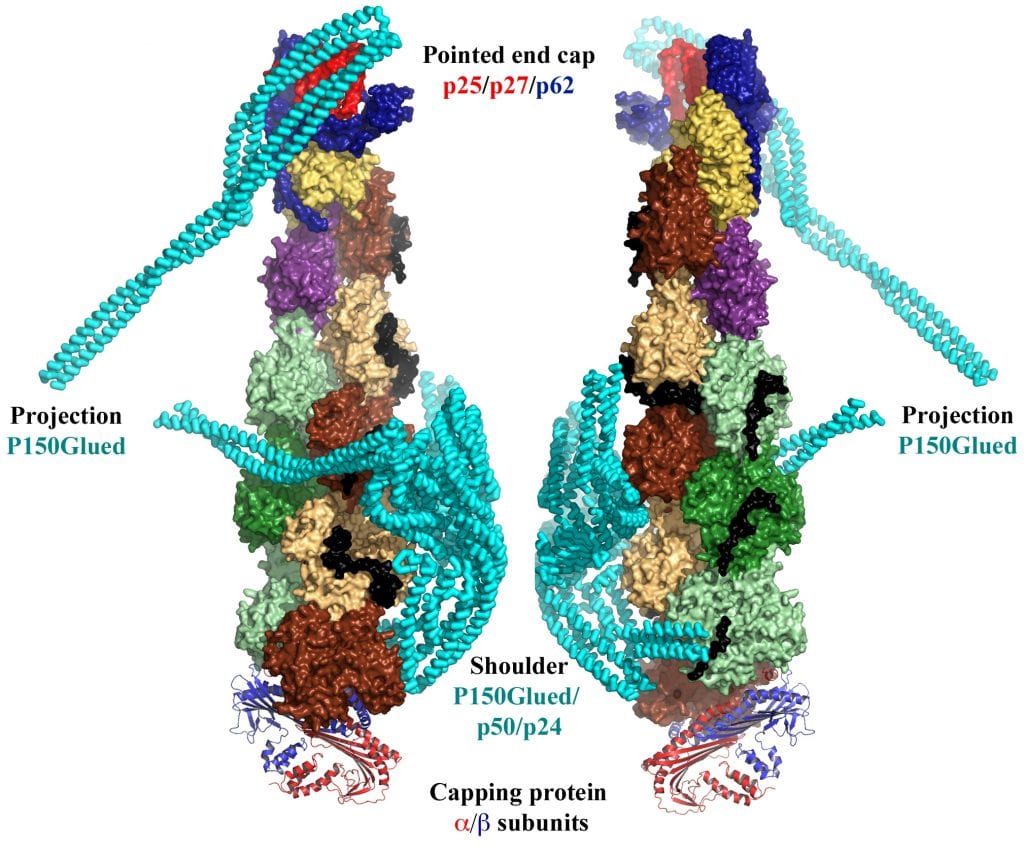
One example of single particle reconstruction is the dynactin complex (see figure below for two views of the 4.0 Ȧ resolution of this complex). This minifilament is constructed from ten actin-like protomers: eight protomers of the actin-related protein 1 (Arp1, shown in shades of green and brown), one protomer of Arp11 (represented in yellow) and one protomer of actin (purple). As can be seen in the figure, this minifilament is capped at each end by a variety of different proteins. In addition, the shoulder complex – composed of p150Glued, p50 and p24 (shown in cyan) – binds to the side of the minifilament. p150Glued links the cargo-binding dynactin filament to the microtubule motor dynein to transport large cargos such as organelles and vesicles.
The authors also discuss how cryo-electron tomography can be used to image physiological structures within cells and how the combining several techniques leads to higher resolution structures and therefore to a better understanding of the respective complex.