Recent advances in microfabrication techniques have allowed for the development of miniature, implantable electronics. When combined with soft materials, these implantable electronics can be used in applications such as stretchable and flexible biosensors. Important requirements for the use of biosensors include wireless monitoring capabilities, and manufacturing reliability and scalability. Until now, implantable electronics often have limited readout distances and rely on slow, low-throughput fabrication procedures. They are also often ridged and bulky, making them incompatible for soft tissues or blood vessels.
A challenging implant location is in the cerebral arteries. Aneurysms exist in these contoured and narrow arteries in 6% of the population, making the development of effective treatments and follow-up monitoring critical. In the vascular system, implantable sensors must be stretchable and flexible while maintaining a low profile in order to not disrupt blood flow.
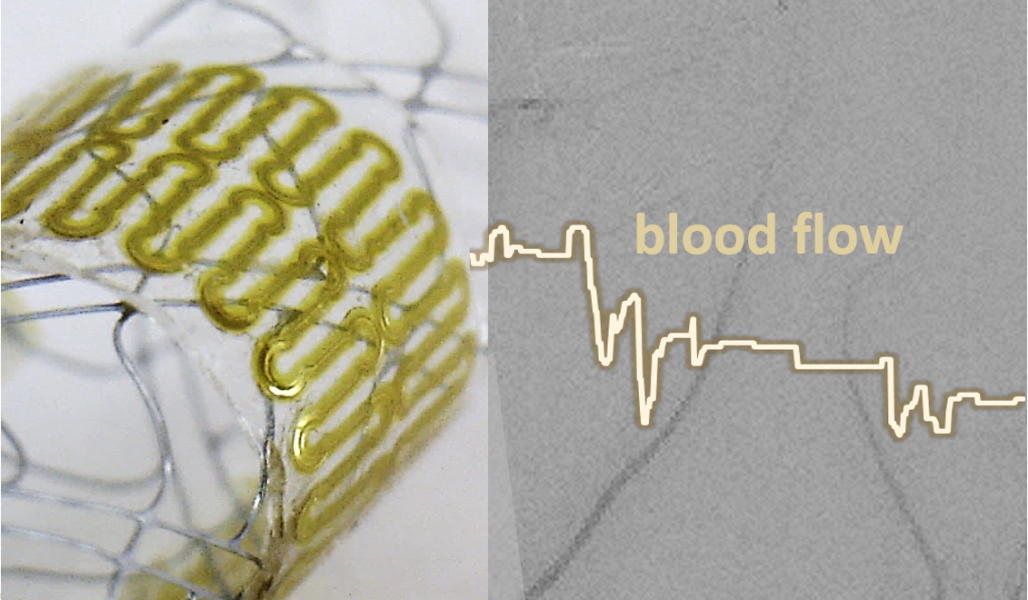
In a study recently published in Advanced Science, Robert Herbert, Woon-Hong Yeo, and co-workers from the Georgia Institute of Technology and Hanyang University introduce a fully passive, wireless, low-profile capacitive sensor and coil inductor with enhanced readout distance fabricated via aerosol jet printing (AJP).
To fabricate the sensor, AJP was used to print four aligned layers followed by seamless integration onto a soft elastomer. In short, the sacrificial polymethyl methacrylate (PMMA) layer was spin-coated on a glass substrate, followed by a polyimide (PI) layer (dielectric layer) for transfer, which was then cured.
A silver nanoparticle (AgNP) ink (capacitive electrode) was then printed and sintered on a plasma-treated PI layer, followed by printing of a second PI and Ag layer. The PMMA was then dissolved in an acetone bath for transfer to the elastomer.
This integration process enables the sensor to conform to stents with high flexibility and stretchability and can be deployed via conventional catheter procedures.
A new inductive coupling method was also optimized and used to monitor hemodynamics via a wireless detection method at distances that surpass existing devices (6 cm). As a proof of concept, the method was tested through air, saline, beef, and in two biomimetic cerebral aneurysm models.

Mechanics of the printed biosensor for catheter-based deployment in a target vessel.
The system demonstrates significant improvement in both implantation size and readout distance compared to other systems. The inductive coupling method is also an improvement in terms of the ratio of wireless readout distance and sensor cross-section area. The wireless distance allows for the integration of the system with cerebral aneurysm sensors for monitoring hemodynamics in the brain, which require an average readout distance of 3–6 cm.
Currently, a restraint of the system is the integration of an implantable coil with the stent and sensor to achieve a complete implantable package, which the team hopes to realize in the near future.