Since their invention in the 1970s, lithium-ion batteries (LIBs) have become a cornerstone of modern society, powering everything from personal portable electronics to space satellites. This technology has made such an impact in shaping the world that its inventors, John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino received the Nobel Prize in Chemistry in 2019.
There is still significant ongoing research in improving LIB performance, as they are not only essential to meeting the requirements of the devices of the future — ones that are smaller and cheaper, with substantially longer battery lives and faster charging capabilities — they are also critical to realizing a more sustainable and greener society.
Emerging technologies that rely on batteries, such as electric vehicles and grid-scale energy storage for renewable energy sources have the potential to reduce society’s dependence on fossil fuels and combat climate change. However, these applications all require batteries with higher energy densities and/or faster charge and discharge rates than are currently available.
The energy and power densities of conventional LIBs that use planar electrodes are often coupled. This means that for a given footprint area, increasing the mass of an electrode will increase the energy density, but the resulting increased electrode thickness will reduce the power density due to the longer distance that the ions and electrons must travel.
If this relationship could be decoupled, then the energy and power densities of the LIB electrodes could be simultaneously improved. 3D architecting of an electrode material allows for thick electrodes to be comprised of micro and nanosized constituents, effectively increasing the mass loading of the electrode without the problems associated with longer transport lengths.
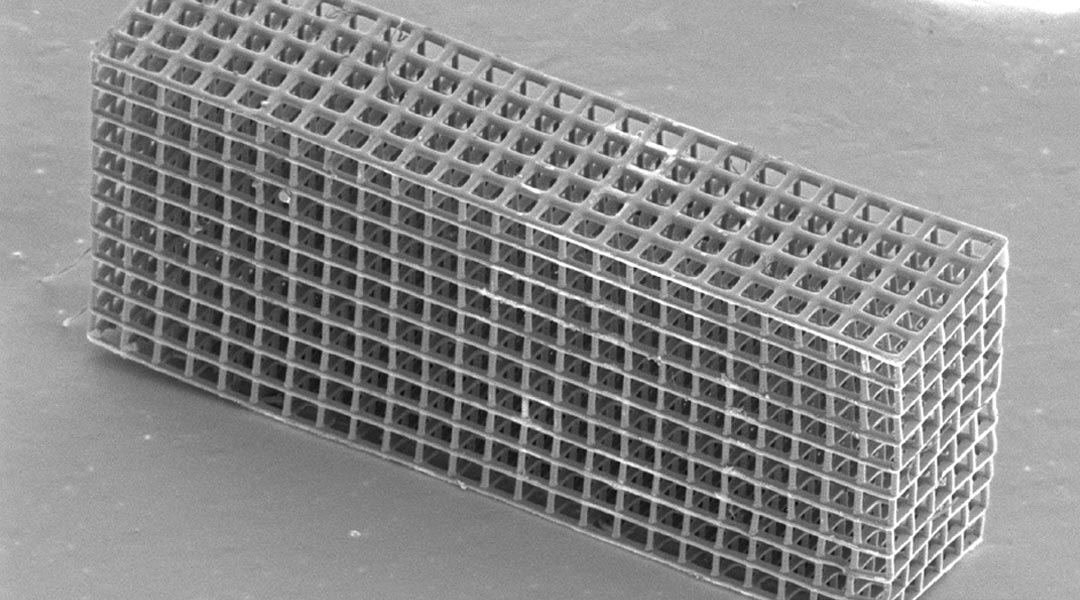
Additive manufacturing is a promising route to fabricating these types of 3D architected electrodes and has been explored extensively in recent years. However, almost every demonstration of this process so far has revolved around the extrusion of nanoparticle inks, which are limited in terms of the resolutions and geometries that can be achieved. Vat photopolymerization techniques offer better resolutions and are able to access more complex geometries but have struggled to fabricate nonpolymeric electrode materials.
In two articles recently featured on the covers of Advanced Energy Materials and Advanced Materials Technologies, a team of researchers led by Professor Julia R. Greer at the California Institute of Technology (Caltech) developed two facile methods for fabricating carbon and lithium cobalt oxide structures using digital light processing printing, and showed that these materials could be used as 3D LIB anodes and cathodes respectively.
Central to both methodologies is the use of thermal post-processing treatments to convert a 3D printed polymer into the desired electrode material. “Pyrolysis of polymers has been known to result in the formation of carbon”, explains Kai Narita, a graduate student from the Greer group and lead author of the study in Advanced Energy Materials. “Our approach exploits this phenomenon to fabricate 3D carbon materials. We simply use a commercially available photoresin with digital light process printing to create 3D polymer structures, which we then pyrolyze at 1000˚C to convert into carbon ones.” The team then showed that these 3D carbon materials could function as anodes in a LIB, reporting excellent performance and stability, and also showing how transport lengths in the electrode affect rate performance.
To make the cathode material reported in Advanced Materials Technologies, the researchers designed a novel 3D-printable polymer system that could be converted into lithium cobalt oxide after thermal treatment. Solution combustion synthesis is a well-established materials chemistry technique to make metal oxides via the combustion of metal nitrates. The team combined this with digital light processing printing by designing a 3D-printable hydrogel system that contained dissolved metal nitrates. Thermal treatment of these hydrogels would then initiate the combustion reaction to yield metal oxide structures . By incorporating lithium nitrate and cobalt nitrate into the 3D printed hydrogel, they could be calcined in air at 700˚C to produce the lithium cobalt oxide structures. These materials were shown to be electrochemically active and could operate as 3D cathodes in a LIB. The study also highlighted the impact that the polymer composition had on the final microstructure and chemical composition of the calcined lithium cobalt oxide.
These studies show that vat photopolymerization of battery-relevant materials is now not only possible but can be achieved in a facile way using commercially available reagents and simple thermal treatments. Moving forward, the team is utilizing these techniques to study fundamental questions about how electrode architecture, such as topology and relative density, impact battery performance.
That knowledge can then be leveraged to design better 3D anodes, cathodes, and solid electrolytes, which can then be combined together to create fully 3D all solid-state LIBs with enhanced performance. “Creating 3D-sculpted electrodes, with full control over their architectural design, dimensions, and now – materials, is bringing us even closer to the eventual attainment of the Holy Grail, i.e. a scalable and reliable fabrication methodology of solid-state batteries that are safe, mechanically robust, and efficient,” says Professor Greer, who was inspired and encouraged by these research results. In the long run, Professor Greer envisions using these techniques to investigate how architecture can be used to improve other systems that require functional materials, with potential applications in catalysis, actuation, and photonics.
Written by: Daryl Yee
Co-authored by: Kai Narita and Max Saccone
References: K. Narita et al, 3D Architected Carbon Electrodes for Energy Storage, Adv. Energy Mat. (2021) DOI: 10.1002/aenm.202002637
D.W. Yee et al, Hydrogel‐Based Additive Manufacturing of Lithium Cobalt Oxide, Adv. Mater. Technol. (2021) DOI: 10.1002/admt.202000791