Tissue regeneration that aims to restore the function of the damaged or diseased tissue or organ is one of the fastest growing advancement in science with potential of new advancements in medicine. The technology involves using a biomaterial as scaffold, which can imitate the natural tissues or organs and trigger regenerative processes.

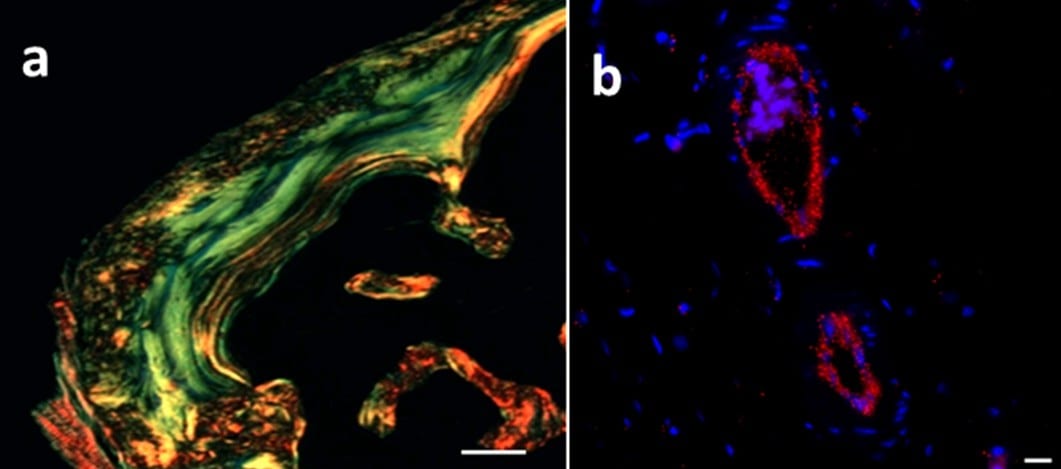
Histological examination of ectopic bone harvested after 7 weeks post implantation showing (a) organized collagen matrix and (b) angiogenesis.
When implanted inside the body, with suitable biological factors, this material stimulates the body to regenerate new tissue by recruiting the individual’s own stem cells. The biodegradable material is engineered in such a way that scaffold simply ‘melts away’ synchronized with the regeneration process. This controlled degradation of the scaffold is the key for successful development of regenerative medicine.
One of the most intriguing challenges in designing hydrogel scaffold for biomedical applications is to obtain good in vivo stability with controlled degradation. The best way to achieve this is by engineering natural extracellular matrix mimetic scaffolds that has unique degradation characteristics utilizing ubiquitous enzymes present in our body. However, stability of such materials require smart synthetic chemistry that is mild enough so that recruited cells do not feel any toxicity and find this artificial scaffold close to its natural environment.
Recently, Dr. Varghese and coworkers from Uppsala University published an article in Advanced Functional Materials where they highlight a versatile synthetic strategy that could facilitate significant improvement of material property by tuning the electronic character around hydrazone linkages. This type of hydrazone linkage between polymers remained resilient to rapid hydrolysis and significantly enhanced the mechanical property and enzymatic stability of this hydrogel.
When they utilized this hydrogel system for delivering recombinant bone morphogenetic protein-2 (rhBMP-2), they found mature bone formation with oriented collagen and blood vessels as natural bone with several folds lower dose of growth factor (Figure 1). This remarkable achievement is indeed phenomenal and shows the potential of such engineered material for various other biomedical applications. The injectability of such materials is equally important for clinical applications, as it is amenable to minimal invasive procedures. The low cost for manufacturing, easy scalability, non-toxicity, and established biocompatibility of hyaluronan-based materials will favor translation of such materials for clinical application.