Currently, there is a strong research focus on mimicking natural systems in order to obtain biocompatible and environmentally-friendly materials. Major efforts are invested in unraveling the diverse functionality observed in proteins. With proteins function is dictated by structure, which in turn depends on the amino acid sequence. Twenty monomeric amino acids offer polar, nonpolar, basic, or acidic functionalities.
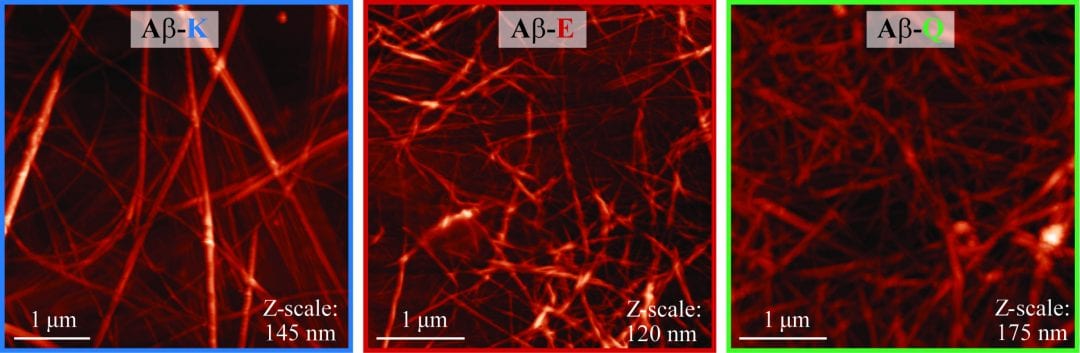
Prof. Nurit Ashkenasy’s group from Ben-Gurion University of the Negev was inspired by biological proton transfer in their recent publication in Advanced Functional Materials. The scientists investigated the effects that single-amino-acid side chains have on the proton conductivity of fibers based on peptides (short synthetic proteins). Introducing a single amino acid of either acidic or basic character into the heptameric sequence of a self-assembling peptide increased proton conduction in the resulting fibers by two orders of magnitude.
The self-doping process is more effective for acidic assemblies compared to basic assemblies, as is evident from a larger concentration of charge carriers, and from a three-fold higher mobility of the formed charge carriers. To know more about this ground-breaking work on self-assembled proton conducting materials read the full paper in Advanced Functional Materials!