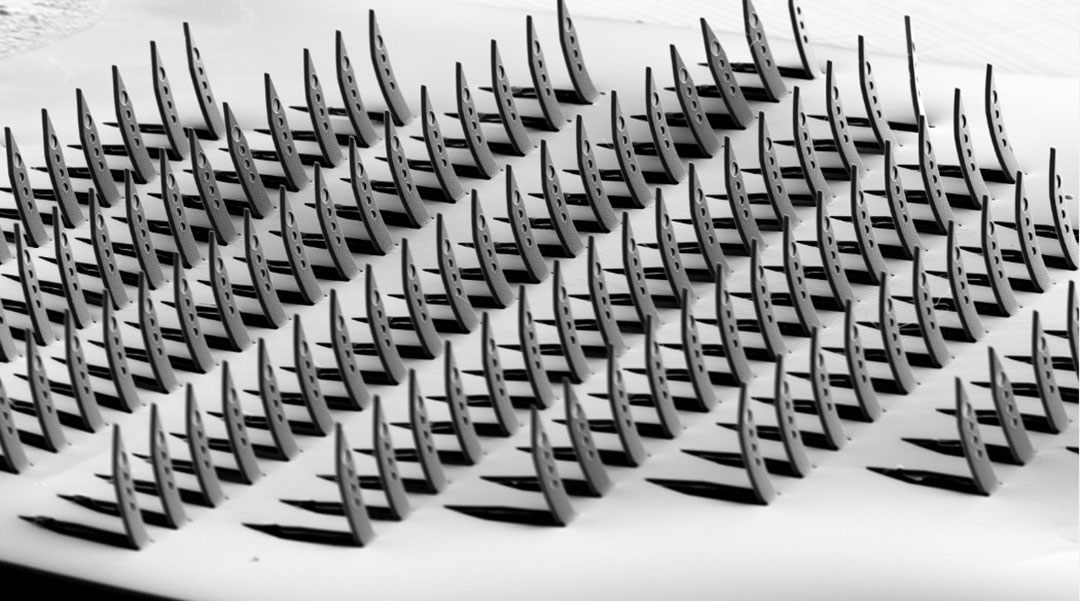
A team of researchers has created neurological probes using a Japanese paper-cutting art form called Kirigama, attempting to match the brain’s complexity by having multiple electrodes that cover all three dimensions in one device.
The desire to understand the complexity of the human brain has led to neuroscience becoming one of the fastest-growing fields of science. Perhaps unsurprisingly, mapping the complex structure of our brains and the neurological function of various regions, and identifying issues with this functioning, is a complex and challenging task.
This task is increasingly being tackled using neuroelectronic interfaces comprising microelectrode arrays (MEAs). MEAs can be employed in studies of cultured neurons outside the brain, or “in vitro,” and in living organisms, “in vivo.” These implants can thus serve both as a bridge between the nervous system and external electronic systems, facilitating the recording and stimulation of neural activity. 3D MEAs can also be used as brain-machine interfaces, not only reading brain signals but also sending signals back, helping better understand how the brain works, study neural disorders, or even develop therapies based on electrical stimulation.
Kirigama transforms a flat sheet of paper into a three-dimensional design, often with an intricate and elaborate structure, through cutting and folding. The creation of the Kirigama-inspired platform is detailed in a paper published in Advanced Materials.
“Due to its 3D design, our device combines the features of two technologies into one. It can record brain signals both from the surface and from deeper layers of brain tissue,” said team member Viviana Rincón Montes of the Forschungszentrum Jülich.
Mapping the complexity of the brain
Team member and Forschungszentrum Jülich researcher Marie Jung added that the kirigami shanks that contain the electrodes are very small — just 50 micrometers (µm) wide. For context, a human hair is on average around 75 µm.
“Our mold design allows us to fold and shape the flexible probe manually under a microscope,” Jung said. “It’s like a key lock, where the parts fit together perfectly. Every time I fold a kirigami probe, I am amazed at how simple and fast our method is!”
Jung added that, unlike some other kirigami methods, the team’s approach doesn’t use hazardous or harsh manufacturing processes. Instead, the researchers utilized a mechanical forming process that sandwiched a flat 2D design between two matching molds, then applied pressure and heat. This transformed an ultrathin, flexible sheet, thinner than paper and flexible as plastic wrap (cling film), into a fully 3D structure on the micrometer scale.
“This process allows all the shanks, which contain the electrodes, to fold into an upright position at the same time,” Jung said. “This allows us to fold up to 128 shanks at the same time — fast and reliably!”
Testing a kirigami-based neurological probe
The team tested their 3D MEA in several steps, beginning in the lab with in vitro models before moving on to in vivo testing.
“First, we tested the 3D MEAs in controlled laboratory conditions. This included testing the mechanical properties of the materials, evaluating the reliability of the folding process using optical inspection, and confirming the devices’ functionality through electrochemical tests,” said Rincón Montes. “We also tested whether the devices would be inserted into brain-like materials to assess their suitability for use in neural tissue and conducted tests under accelerated aging to see how long they could survive under body conditions.”
The researchers also tested whether the devices could be inserted into brain-like materials, assessing their suitability for use in neural tissue, and conducted tests under accelerated aging to see how long they could survive under body conditions.
“Next, we moved on to real biological samples, including human brain slice cultures prepared from cortical tissue donated by patients who had epilepsy surgery and the cortex of living rodents,” Rincón Montes added. “In these tests, we applied different types of stimulation to record diverse types of neural signals. For the human brain slices, we changed the chemical makeup of the surrounding fluid to trigger epileptic-like activity. In rodents, we applied both touch and visual stimuli.
“These experiments allowed us to record a wide range of brain signals — from single neurons to groups of neurons- and observe how responses changed across different brain layers.”
The team found that in terms of manufacturing, their method has a 98% success rate after folding and an 80% success rate after the final post-processing steps, such as adding an electrode coating and packaging. Though the folding process is currently performed manually by aligning the 2D sheet and pressing by hand, they expect this success rate to improve with automation and when employing techniques like pick-and-place systems or micromanipulators.
“One of the most surprising things we discovered was how well a technique typically used for shaping materials on a large scale works at the micro-scale,” Vivana Rincón Montes said. “Our designs offer stability during manufacturing, allowing us to implement them manually without needing complex setups. Moreover, the robustness of our devices has been proven to withstand both mechanical stresses during insertion and biological stresses (e.g., immune responses). In fact, after being implanted for a month, our devices kept their 3D shape when explanted!”
The team is currently working on several ways to improve their 3D MEA devices by refining their process and designs to reach deeper layers of the brain with a single insertion while avoiding blood vessels, thus making the insertion safer. The team is also working on the stability of the electrode coating of their devices by optimizing the formulation and coating methods for conductive polymers combined with noble metals such as iridium oxide.
“Currently, we can create a device with up to 128 shanks, each containing four electrodes, for a total of 512 electrodes,” Rincón Montes said. “However, we can only connect up to 32 electrodes or 64 if we use large, bulky connectors. That’s why we are also working on new ways to connect all the electrodes to external electronics without making the device too big or heavy.”
Rincón Montes added that it may still be some time before 3D MEAs like those of the team are employed in real-world healthcare.
“We need to make sure that the devices are reliable and safe for long-term use, and more research is needed to fully understand how they can help restore lost neural functions,” Rincón Montes concluded. “We are currently focusing on using this technology in visual prosthetics. Thanks to the large number of electrodes, it can support two-way communication — in other words, we can both read and send neural signals, either in the brain or the retina.
“This could open the door to new ways of restoring vision in people who are blind.”
Reference: V. Rincón Montes., M.Jung., J. Abu Shihada., et al., Flexible 3D kirigami probes for in vitro and in vivo neural applications, (2025). DOI: 10.1101/2024.11.05.622167