(Copyright Shaanxi Normal University, used here with permission. Please click here to watch the video on Youku if you are unable to access Youtube)
“Like dissolves like” is a simple yet handy rule that chemists use to tell how well a solvent can dissolve a solute. Established on some of the fundamental principles in chemistry, this rule actually finds viable analogies across the entire discipline, and now a new addition is rolling out from the field of protein crystallization.
Obtaining high quality protein crystals is a key step in structural biology studies, but it could be biochemists’ worst nightmare as well. Many of these biomacromolecules would only crystallize in the presence of specific additives that take laborious jobs to find out, let alone those refusing to give crystals at all. Recently, Peng Yang and his team at Shaanxi Normal University (Xi’an, China) have developed a superhydrophobic coating technique based on the lysozyme fiber network, which provides a facile platform for protein crystallization.
The researchers have previously discovered that lysozymes could quickly assembly into a necklace-like fiber network from buffer under a mild chemical stimuli (Macromol. Biosci. 2012, 12, 1053), readily attachable onto a variety of material surfaces including metal, polymer and inorganic materials. The attachment on material surfaces results in a stable coating that could be directly used or subjected to further functionalization (Adv. Mater. Interfaces 2015, 2, 1400401, front cover). Later, they have also found that the coating carried positive charges, which could capture/release the negatively charged giant unilamellar vesicles (GUVs), providing a new approach towards the system of the controllable capture/release of cells (Soft Matter, 2015, 11, 3094, back cover).
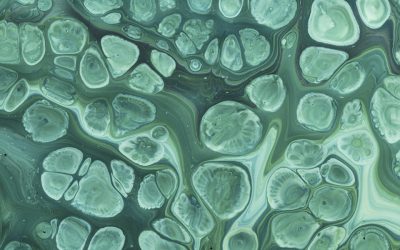
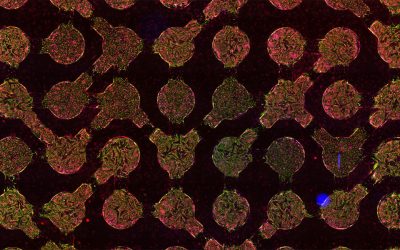
Based on the above findings, the researchers further hydrophobized the densely assembled lysozyme coating by low surface energy molecules, leading to excellent mechanical stability towards external peeling and thermal resistance in the temperature range of -196-200 ℃. They demonstrated for the first time that a protein-based superhydrophobic surface could largely accelerate and facilitate the protein crystallization process. With this platform, protein crystals can be grown on a large scale, even at low concentrations and without the use of additional precipitation/toxic reagents. Moreover, even protein crystal arrays are conveniently attainable by combining protein spotting and crystallization altogether on the superhydrophobic surface.
As a simple and tunable strategy to create functional superhydrophobic surfaces, it holds the great potential to provide a paradigm-shifting solution for protein crystallization, bringing huge impact to related fields, including structural biology, biomedicines, and chemical biology. In addition, such durable and versatile superhydrophobic surfaces are highly sought after in many other emerging applications as well.