As an architect, Gizem Gumuskaya was always fascinated by self-organization. When designing a city, buildings are constructed according to plan, but the patterns its citizens inevitably fall into — where they tend to travel, how they meet, what they do — emerge on their own.
While pursuing a Master’s degree in architecture at MIT, she stumbled into the field of synthetic biology, where she found scientists who were “building bricks from living cells, harnessing what only nature has to offer”.
Now a Ph.D. candidate in biology at Tufts University and the Wyss Institute for Biologically-Inspired Engineering, Gumuskaya applies her architectural instincts towards building robots out of living cells.
In a study published in Advanced Science, her team, led by developmental biologist Michael Levin at Tufts University, transformed cells from the human lung into living, self-propelling robots. These biological machines, called anthrobots, swarm together to help torn tissue heal itself.
In the future, microscopic robots made out of a patient’s own cells may be able to work inside the body to repair damage, scope out signs of disease, or fight off infections.
Building biobots
Anthrobots were not the lab’s first foray into building biological robots or biobots. Nearly four years ago, Levin’s team built Xenobots — controllable, multi-cellular blobs made from embryonic frog stem cells. These blobs were the first fully-biological robots made by stitching cells together into pre-designed structures that could scoot around on their own.
While groundbreaking in the lab, frog-derived biobots have limited therapeutic potential in humans. Foreign cells are likely to be rejected by the human immune system, but human cells (especially one’s own) are more welcome. So, Gumuskaya chose to build anthrobots out of cells that normally line the human trachea.
These cells have the advantage of being covered in tiny hair-like propellers called cilia that help push liquid through human airways. Gumuskaya hoped built-in cilia would “harness what nature already does best” and imbue anthrobots with the power to move freely.
Xenobots were fully biological, but still built one by one through a combination of algorithm-aided design, virtual simulation, and painstaking manual construction. Rather than design each bot individually, Gumuskaya’s research team wanted anthrobots to build themselves.
To do this, Gumuskaya started by growing thousands of individual cells in a special 3D matrix for two weeks, where they multiplied and clumped into tiny spheroids. Once mature (but still immobile), she would extract the spheroids from the matrix. “That’s where they start their journey,” she said.
They were then bathed in a special liquid to coax their cilia out. Once the mature anthrobots, comprised of roughly 100-1000 cells each, were covered in cilia and ready to mobilize, Gumuskaya analyzed how each bot’s shape determined its movement style.
The resulting anthrobots naturally fell into different “character” tropes, Gumuskaya said. Some wound up small, round, and covered with an even coat of cilia. Others were bigger, squiggly, and sported more sporadically-distributed cilia.
These distinct forms lent different anthrobot types their own flavor of movement, with some swimming in a straight line or in a circle, and others mostly wiggling in place. The strong relationship researchers observed between a bot’s shape and its behavior will inform future design choices, possibly giving them the power to make bots with specific, controllable behaviors.
Bridging gaps between neurons
One potential real-world role for anthrobots could be serving as microscopic surgeons, repairing damaged tissue inside the body. To test this capability, Gumuskaya used a standard model for tissue damage: a petri dish of human neurons, scratched down the middle.
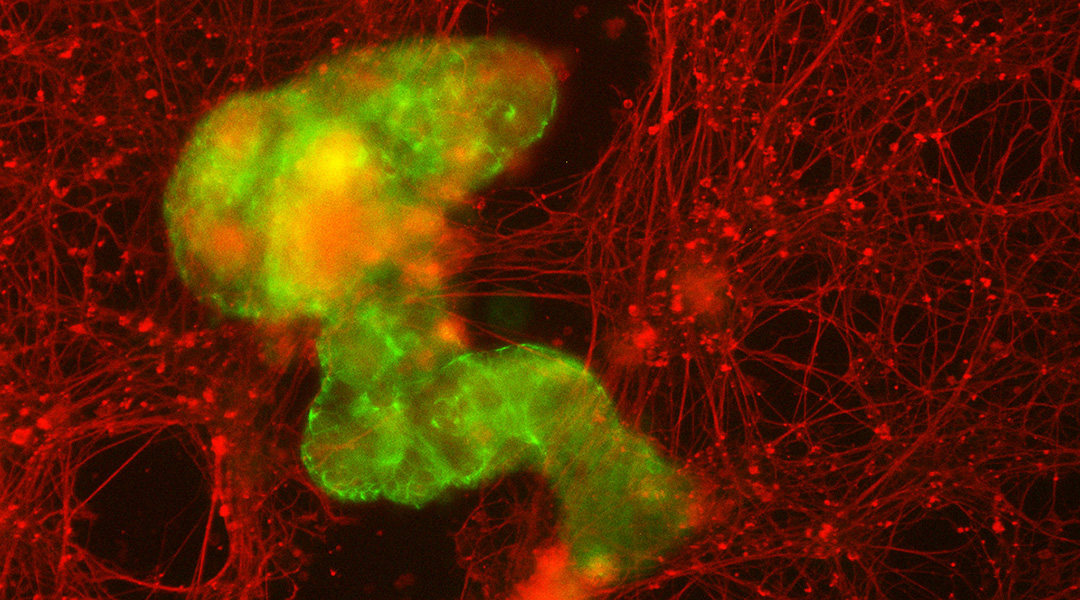
When placed in this environment and allowed to move freely, the anthrobots moved across the scratch. Remarkably, the bots grouped together on their own, forming “superbots” that bridged the torn sides of the damaged tissue, enabling the neurons to regrow underneath them.
The bots’ ability to assemble themselves into larger clusters, Gumuskaya said, is crucial for their potential role in healing human tissue. “When you put these larger structures into neuronal tears, the collective accomplishes something that an individual couldn’t.”
While one tiny anthrobot on its own might slip through a scratch in tissue, a larger superbot could fill in the gap, allowing the wounded tissue to reseal itself.
Moving forward, the team hopes to test anthrobots in more realistic settings to see how they might handle repairing more complex injuries, beyond a single scratch, and to see which anthrobot behaviors are most helpful in getting the job done. Someday, these bots may even serve as a form of personalized medicine, using a patient’s own cells as raw material for custom tissue repair.
“As a designer, as an engineer, you can create things that evolution didn’t have any reason to create,” Gumuskaya said. “Why restrict ourselves to what’s already out there?”
Reference: Gumuskaya et al., Motile Living Biobots Self-Construct from Adult Human Somatic Progenitor Seed Cells, Advanced Science (2023). DOI: 10.1002/advs.202303575