Medicine is advancing at an increasingly accelerated rate, and new drugs and therapeutics are being developed every year. Surprisingly, one of the greatest menaces in public health today is not the availability of treatment options, but their imprecise implementation. In addition to drugs having negative side effects, relying on patients to rigidly follow their prescribed medication schedules has been demonstrated to significantly reduce effectiveness. Drug-delivering implantables could eliminate the issue of patient adherence to strict medication schedules and provide intervention exactly- and perhaps even only- when needed. For instance, chronotherapy is a recent research area which focuses on when in the day medications are taken, which, in some cases, heavily impacts efficacy (i.e., orally ingested birth control, glucose). Additionally, direct implantation can minimize or eliminate the serious side effects of necessarily evils, such as chemotherapy, by localizing its presence within the body to the malignant region.
Long-term implantable technologies that provide controlled drug release are in their infancy, as progress is severely limited by the manufacturable and clinically relevant size, biocompatibility, and durability within the body. Furthermore, utility goes hand in hand with sophistication, which is hampered by the size of batteries and power draw of remote communications technologies, among others. Happily, matching and melding the newest developments in robotics, microprocessing, and tissue engineering have enabled miniaturization of implantable devices. While most research is still focusing on microscale interactions, nanoscale devices are versatile alternatives for high impact problems, such as brain cancer, mental disorders, and vascular issues, among others.
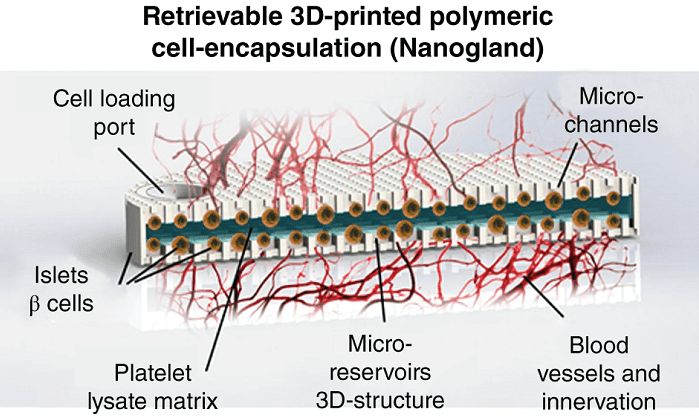
A review article recently published in WIREs Nanomedicine and Nanobiotechnology covers nanofluidic implantable technologies, broken out by fields of diagnostics, biosensing, therapeutics, and theranostics – which is a combination of sensing and therapeutics. The latter is an exciting new direction, wherein an ideal theranostic system can seek out a harmful chemical or cell type, identify it, and provide on the spot treatment. In general, nanofluidic systems have unprecedented potential for developing personalized, point‐of‐care medical technologies. Combination therapies, chronotherapy, and lab-on-a-chip technologies give doctors and their patients a far greater degree of flexibility in determining and achieving the best care, with devices correctly sized for administering medications without harming or inconveniencing the patient. Nanofluidic implantable systems will enable these advantages while being compatible with both current and future drugs. As commensurate advances in power density and electronic processing are continually redefining the possible, the field is expected to grow into an advanced toolset for preserving human health.
Text kindly provided by the authors.