Hydrogels make up a large portion of biomedical materials, such as contract lenses, drug delivery carriers, and three-dimensional (3D) cell cultures. Chemically crosslinked poly(ethylene glycol)s (PEGs) are the most common source of artificial hydrogels, and many of its chemical and physical properties can be tuned by the degree of crosslinking.
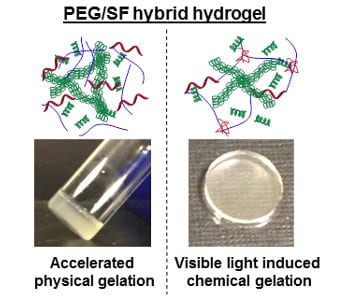
Chien-Chi Lin and co-workers at Indiana University-Purdue University Indianapolis (USA) have previously developed a gelation method based on thiol-acrylate photopolymerization to fabricate dually degradable (i.e., proteolytic and hydrolytic) hydrogels. However, they have also found it challenging to make PEG-based hydrogels with high stiffness and fast degradation rates, both of which are desirable for certain biomedical applications, as these two factors are coupled to each other in chemically cross-linked hydrogels. Now, with their collaborators in the Rural Development Administration from the Republic of Korea, the team has elegantly solved this dilemma by physical entrapment of silk fibroin (SF), a silk worm-derived natural protein that can physically self-assemble into higher order fibrillar structures, which has recently appeared in the Journal of Applied Polymer Science.
The strategy involves visible light mediated curing of thio-acrylate PEG hydrogels in the presence of interpenetrating SF fibrils. The obtained network is thus composed of non-degradable linear polymer chains, hydrolytically labile thioether ester bonds, and embedded with silk fibrillar matrices. The researchers have systematically studied the effect of SF entrapment, and find that doping with sonicated SF increases gel moduli while barely affecting the hydrolytic degradation rate. The combination of the chemical and physical approaches provides unprecedented multifaceted control in modulating the properties of hybrid hydrogels.
This study opens the access to a hydrogel platform suitable for a wide variety of biomedical applications, such as drug delivery and tissue engineering. In the future, the researchers will further “characterize the potential formation of fibrillar silk in the chemically crosslinked PEG-based hydrogels” while “using this material platform to design artificial tumor niches for studying pancreatic cancer cell growth, invasion, and drug responsiveness”, as Dr. Lin envisions. In addition, the team is also “interested in exploring the incorporation of SF with different architectures (e.g., microspheres, fibers, porous scaffold, etc.) to afford a hydrogel with tunable degradability and anisotropic biophysical properties”.