Technological advances have revealed that the seemingly equivalent cells of the early embryo display a surprising degree of variation in the expression of cell type specific genes. This cell-to-cell heterogeneity primarily originates from the inherently noisy processes underlying gene expression. How do cells make reliable cell fate decisions to generate the correct ratios of cell types despite this noise?
Understanding how individual cells decide to differentiate into specialized cell types is a fundamental goal of developmental biology. The first decisions cells make in the mammalian embryo is whether to develop into embryonic cell types or extra-embryonic tissues that support embryo growth during gestation. The embryo’s survival critically depends on generating the correct proportions of extra-embryonic and embryonic cell types.
Technological advances have revealed that the seemingly equivalent cells of the early embryo display a surprising degree of variation in the expression of cell type specific genes. This cell-to-cell heterogeneity primarily originates from the inherently noisy processes underlying gene expression. How do cells make reliable cell fate decisions to generate the correct ratios of cell types despite this noise?
A review of recent progress in this field published in WIREs Developmental Biology, argues that intercellular communication provides a mechanism to exploit and buffer cell-to-cell variability.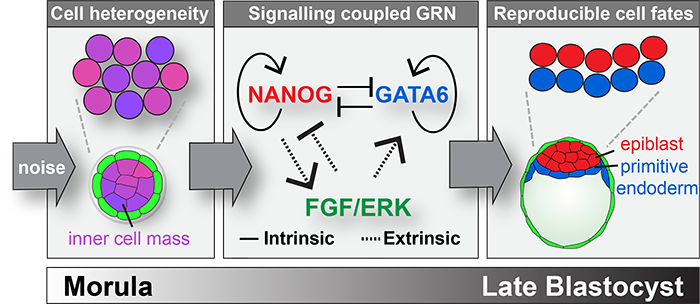
Several mathematical models have been developed to explain the mechanisms underlying fate decisions in the inner cell mass, a population of cells that gives rise to both extra-embryonic (primitive endoderm) and embryonic (epiblast) cell types.
These theoretical approaches are based on the knowledge of the genes that control fate decisions and model how noise can generate reproducible outcomes across populations of cells. Cell fate decisions within the inner cell mass display properties of a self-organizing system: cell communication (via FGF/ERK) transforms the noisy expression of antagonistic genes that direct cell fates (Nanog, Gata6) into the reproducible formation of the mammalian blastocyst stage embryo. Such theoretical frameworks help identify general strategies of cellular decision-making, and highlight the importance of biological inputs (i.e. noise) that are difficult to assess experimentally.
Kindly contributed by Claire Simon.