In contrast to e.g. reptiles where the sex of the animal is usually determined by the environmental temperature during embryonal development, vertebrates are characterized by a genetic sex determination system. For the vast majority of mammals, this implies an XY male and XX female sex chromosome system. While guaranteeing independence from environmental factors, this system carries the disadvantage of an imbalance in gene dosage between females who possess two X chromosomes and males who only have one. This imbalance is resolved by the so-called X (chromosome) dosage compensation.
While X chromosome dosage compensation has already been well characterized in somatic cells, in their review published in BioEssays, Mahesh Sangrithi and James Turner discuss this phenomenon with a particular focus on the germ line. The authors first briefly review the mechanisms of mammalian sex determination as well as sex chromosome evolution before taking a closer look at the mechanisms of X chromosome dosage compensation.
The large difference in size and gene content between the X and Y chromosome leads to an imbalance in the dosage of X-linked versus autosomal genes. In order to counteract this imbalance, two mechanisms for X dosage compensation exist: X chromosome upregulation (XCU) and X chromosome inactivation (XCI). XCU in males ensures dosage parity between the X and autosomes by upregulating X-linked gene expression two-fold. However, in females this would result in two-fold excess of X- over autosomal products. Therefore, in females, XCI leads to the silencing of one of the X chromosomes. It is important to note here, however, that not all X chromosomal genes are dosage sensitive and therefore not all of them are affected by XCU or XCI.
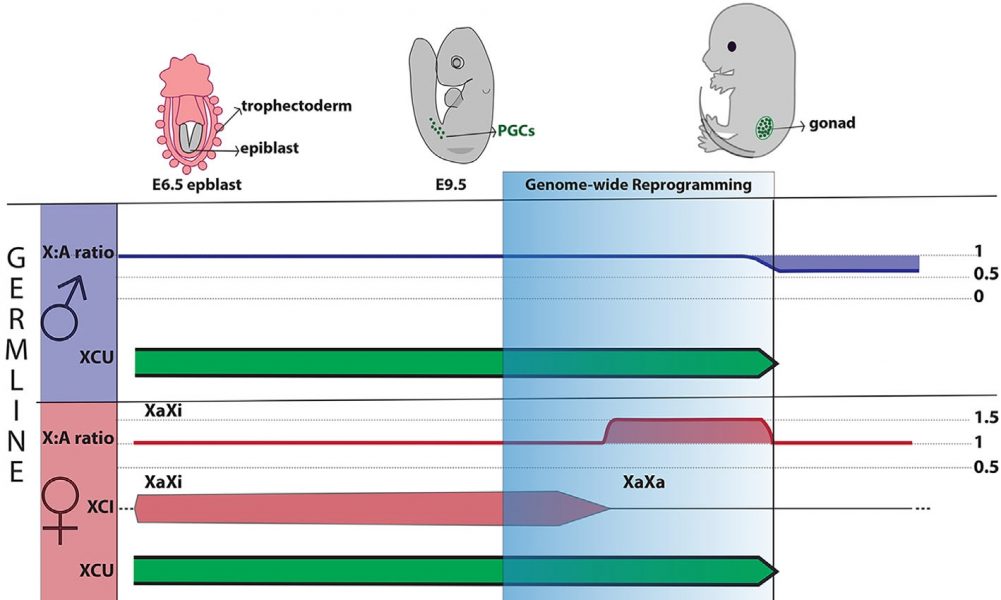
In the following, Sangrithi and Turner review X dosage compensation states in the germ line. In mice, germ cell development initiates around embryonic day 6.5. At around embryonic day 10.5. to 11.5 the primordial germ cells undergo genome-wide reprogramming. During this stage, in female mice loss of XCI is followed by loss of XCU resulting in an X chromosome to autosome expression ratio larger than 1 (X:A ratio > 1). This state of X-hypertranscription lasts for about three days before the ratio is corrected back to one. In male mice, loss of XCU also takes place leading to a state of X dosage decompensation (X:A ration < 1). This clearly shows that germ cells exhibit X dosage compensation states that not only differ between the sexes but are also different from somatic cells.