In non-muscle tissues in the body, the stiffness of the tissue (E) dominates tissue biomechanics, i.e. the work done by a cell (when it contracts) goes towards deforming the surrounding matrix and cells. Consistent with this fact – previous studies using, for example, hepatocytes, observed optimal hepatocyte function when they were cultured on materials with a stiffness comparable to that of the liver. However, the heart differs from other organs in the body because it is a pump. Therefore, during a contraction cycle, in addition to overcoming tissue stiffness, work has to be done to push out the blood contained within the heart against the external resistance.
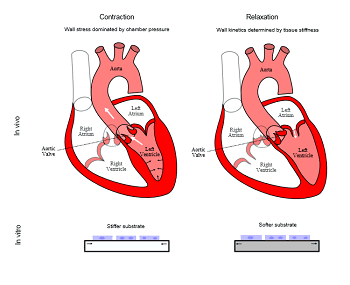
 Dr. Domian and his colleagues now present data which demonstrate that the pressure in the heart wall required to pump blood dominates the biomechanics of the contraction cycle of the heart. Conversely, the kinetics of the relaxation of the heart wall is indeed governed by wall stiffness. Thus, they argue, this system demonstrates the inherent challenges of trying to imitate a chamber (the ventricles of the heart) using 2-dimensional or solid cuboidal 3-dimensional constructs (do not contain a cavity). For such latter systems, since the chamber pressure varies with the development stage, as well as over the cardiac cycle, optimal materials may be dynamic ones in which the stiffness can be modulated over slow and fast time scales.
Dr. Domian and his colleagues now present data which demonstrate that the pressure in the heart wall required to pump blood dominates the biomechanics of the contraction cycle of the heart. Conversely, the kinetics of the relaxation of the heart wall is indeed governed by wall stiffness. Thus, they argue, this system demonstrates the inherent challenges of trying to imitate a chamber (the ventricles of the heart) using 2-dimensional or solid cuboidal 3-dimensional constructs (do not contain a cavity). For such latter systems, since the chamber pressure varies with the development stage, as well as over the cardiac cycle, optimal materials may be dynamic ones in which the stiffness can be modulated over slow and fast time scales.
The researchers also point out that with regard to engineered systems, an additional variable must first be assigned. What does the experimenter/engineer desire, maximum force, shortening, maturation rate, or something else? Current data demonstrates that the optimal stiffness for each of these may be different. Thus, the optimal elastic modulus for cardiac tissue engineering cannot be determined a priori. Rather, it must be determined experimentally.