Membrane nano-tomography in living cells: Label-free evanescent microscopy enables full-field and real-time tracking of membrane processes without signal fading and cell perturbation.
 Membranes play a pivotal role in numerous cell mechanisms, in particular for internalization, adhesion and motility studies. In terms of optical imaging of the membrane, special configurations are needed to remove the light coming from the inner part of the cell. French scientists now show that through-the-objective evanescent microscopy (epi-EM) is a powerful technique to image membranes in living cells.
Membranes play a pivotal role in numerous cell mechanisms, in particular for internalization, adhesion and motility studies. In terms of optical imaging of the membrane, special configurations are needed to remove the light coming from the inner part of the cell. French scientists now show that through-the-objective evanescent microscopy (epi-EM) is a powerful technique to image membranes in living cells.
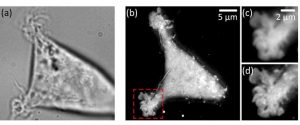
In label-free evanescent microscopy (EM), configurations similar to total internal reflection fluorescence (TIRF) have been proposed: prism-based or through-the-objective. However, in the latter case, these evanescent techniques have not spread much, with a relative preference for the prism-based configuration also called total internal reflection microscopy (TIRM). The team led by Pierre Bon chose the through-the-objective based configuration (epi-EM), which enabled super-axially resolved tomographic reconstruction of the basal membrane of label-free living cells. The implementation of epi-EM only required an easy to settle illumination/collection scheme on a standard inverted microscope. Only a high-NA objective (NAobj > 1.33) was needed for living biological sample studies and a spatial filter on the epi-illumination arm in order to reject under-critical angle illumination.
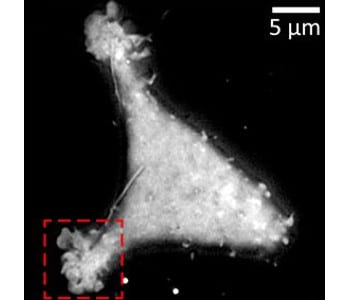
Either bead calibration or a multilayer Fresnel model could be used to retrieve nanometric position. Based on a multilayer Fresnel model, the team was able to retrieve the membrane/interface distance with 10 nm precision. The researchers applied this nano-axial tomography to retrieve quantitative information on invagination dynamics of living cell membranes. They studied the membrane elevation map of living cells (Wt HEK-293) during 15 minutes at one frame per second without perturbing the sample.
The results demonstrate that epi-EM gives easily access to axially super-resolved images of unlabeled microscopic samples with almost no microscope modification, and at least a doubled lateral resolution compared to classical TIRM. A study can be of any duration as the signal level is not sensitive to any fluorophore stability dependence and the photoxicity is very low as barely any light is absorbed by the sample. The scientists are convinced that this technique will be useful for cell motility and adhesion studies when the sample cannot be modified (ex. stem cells) or when very fast and/or long studies are required.