RNA editing, an important post-transcriptional mechanism, leads to specific nucleotide changes at the RNA level. In the most common type of RNA editing adenosine is changed to inosine, a step that is mediated by ADAR enzymes. A second, but much less common type, is C-to-U editing that requires the deaminase APOBEC enzyme. When such an exchange happens at the level of messenger RNA (mRNA), the amino acid sequence in the resulting protein is altered. However, this editing may also take place in non-coding RNAs, such as microRNAs (miRNAs), where it has a regulatory role.
Yumeng Wang and Han Liang from Baylor College of Medicine and The University of Texas MD Anderson Cancer Center, have taken a closer look at how RNA editing affects cancer, particularly focusing on the role of miRNAs. In their review in BioEssays, they discuss the different mechanisms of RNA editing that may modulate the initiation and progression of cancer, and consider the challenges and future directions of studying this phenomenon.
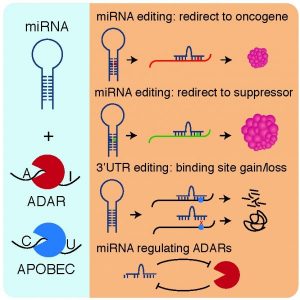
As shown in the figure, the authors identify and discuss four major mechanisms through which miRNAs interact with RNA editing, and how this may affect cancer.
In the first two mechanisms, miRNA editing leads to miRNAs either being redirected to oncogenes or to tumor suppressors. One such example is the miR-376 family. The editing level of miR-376a-5p is dramatically reduced in glioma cell lines. Studies revealed that the unedited form of this miRNA targets Ras-related protein RAP-2A (RAP2A), leading to a reduced mRNA and protein level of RAP2A. This inhibition of RAP2A promotes cell migration and invasion, resulting in more aggressive glioblastomas.
The third mechanism functions via 3’UTR editing in an mRNA leading to the disruption or creation of binding sites for miRNAs. One example here is MDM2, a well-known oncogene in the p53 pathway. MDM2 has binding sites for miR-200b/c in its 3’UTR, and these sites are among the most over-edited in cancer.
In the last mechanism, miRNAs may directly regulate the enzymes responsible for RNA editing. In metastatic melanoma cells, for example, miR-17 and miR-432 are frequently amplified, leading to ADAR1 silencing.