In recent years, a variety of advanced electrochemical biosensors based on nanomaterials with superior properties of charge transport, large surface area and good biocompatibility have been developed into high performance chip-based electrochemical biosensors.
However, most were designed to detect target analysts in solution, i.e., in vitro, lacking the possibility of in situ and real-time sensing of analysts in an in vivo environment. On the other hand, in vivo real-time biosensing has been achieved via metal needles with integrated biosensors, but due to the invasive nature of needle penetration, this method tends to induce undesirable wounds and potential risk of infection, limiting the possibility of long-term in situ sensing.
An ideal biosensor would be minimally-invasive and result in real-time, in vivo transdermal biosensing with high sensitivity.
Recently published in Small, a team of researchers from Sun Yat-sen University, China, has developed a unique real-time, in vivo, and minimally-invasive transdermal biosensing system for H2O2 monitoring, and has successfully demonstrated the system on both pigskins and living mice.
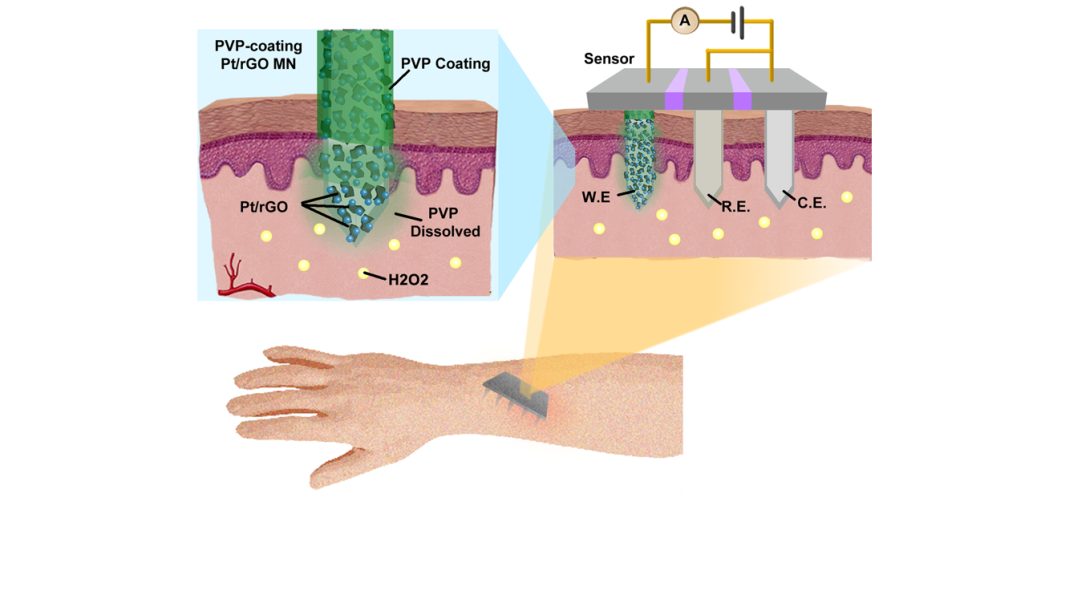
To overcome the challenges associated with biosensor fabrication, the authors used stainless steel microneedles (MNs) integrated with nanohybrids consisting of reduced graphene oxide and Pt nanoparticles (Pt/rGO) as the key components of the electrochemical biosensor.
The Pt/rGO significantly improved the detection sensitivity of the MN electrode, while the MNs were utilized as a painless transdermal tool to access the in vivo environment. Furthermore, the Pt/rGO nanostructures were protected by a water-soluble polymer layer to avoid mechanical destruction during the MN skin insertion process. The polymer layer is readily dissolved by the interstitial fluid and exposes the Pt/rGO on MNs for biosensing in vivo.
This work provides a unique systematic solution for in vivo, real-time biosensing, and takes advantage of the high sensitivity of hybrid nanomaterials and the minimally-invasive nature of an MN patch. Moreover, this solution can be readily extended to sensing a wide range of biomarkers for rapid and continuous disease monitoring, due to the excellent flexible design of the nanostructural sensing modulus.
The next challenges lie in the sensitivity and durability of the system. Biomarkers with low in vivo concentration (< 1 μM) require recorded electrochemical signals with excellent signal-to-noise ratio, placing greater demands on the material structures and system designs.
Due to the one-shot character of the polymer coating, durability is another challenge. The authors hope to introduce an advanced strategy to protect the sensing nanostructures, e.g., a porous polymer layer as a non-dissolvable protection layer, or metamaterials-structure MNs with nanomaterials integrated interiorly, to improve the system in the future.