After an injury, cells are like hard-working carpenters, busily tearing down and rebuilding the materials around them to repair the tissue. Tissue engineers have long dreamed of the day when they could mimic this cellular remodeling. An emerging new type of material may be bringing that dream closer to reality. In the past, tissue engineers often had to rely on materials that cells could only tear down and destroy. As cells moved through the material, they were like a demolition team taking sledgehammers to the walls, resulting in a material that was irreversibly damaged. In contrast, these new materials have molecular “doors” in them that can open and close, allowing the cells to move around without causing permanent damage. These new materials, termed “adaptable hydrogels,” will enable scientists to create new types of engineered tissue in the lab that could be used to repair, replace, or regenerate injured tissue.
After an injury, cells are like hard-working carpenters, busily tearing down and rebuilding the materials around them to repair the tissue. Tissue engineers have long dreamed of the day when they could mimic this cellular remodeling. An emerging new type of material may be bringing that dream closer to reality. In the past, tissue engineers often had to rely on materials that cells could only tear down and destroy. As cells moved through the material, they were like a demolition team taking sledgehammers to the walls, resulting in a material that was irreversibly damaged. In contrast, these new materials have molecular “doors” in them that can open and close, allowing the cells to move around without causing permanent damage. These new materials, termed “adaptable hydrogels,” will enable scientists to create new types of engineered tissue in the lab that could be used to repair, replace, or regenerate injured tissue.
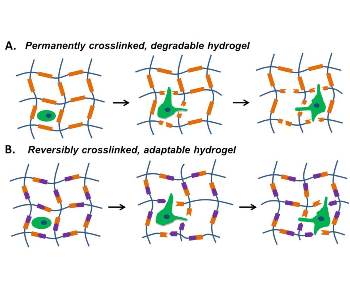
In their review, Huiyuan Wang and Sarah Heilshorn from Stanford University elegantly summarize recent advances in the design of adaptable hydrogels for cell-encapsulation, cell-culture, and cell-transplantation applications. First, critical design criteria for adaptable hydrogels used in tissue-engineering applications are discussed. Following this, they highlight case studies of recently reported adaptable hydrogels, with a focus on the physical and chemical crosslinking mechanisms that can be used. The review closes with a discussion of the challenges involved in using adaptable linkages to build hydrogels and potential future applications of adaptable hydrogels.