Although the medical and pharmaceutical fields have come a long way in diagnosing disease states and producing highly potent drugs, the lack of effective delivery of such therapeutics to the target organ with desired pharmacokinetics remains one of the major challenges in this process.
The advent of nanotechnology, along with advances in protein engineering and materials science, have brought new hope to patients. The impact of nanotechnology on medicine — nanomedicine — is recognized by the development of novel nanoscale therapeutics and diagnostic and imaging modalities.
In a recent review published in WIREs Nanomedicine and Nanobiotechnology, Professor Joerg Lahann and his team from the University of Michigan discuss state-of-the-art nanoparticle drug delivery platforms, their advantages and shortcomings, and future directions towards clinical translation.
“The ability to impart multiple functions to a single delivery system, engineering both bulk and surface properties, provides a means to answer some of the greatest remaining challenges in the field of drug delivery,” said Jason Gregory, a PhD student in the Lahann Lab.
In fact, approaches to address this conundrum include the development of multifunctional particles, cell-mediated transport mechanisms, and the use of biologically derived materials. Multifunctional particles can possess two or more dissimilar properties through surface or bulk anisotropy.
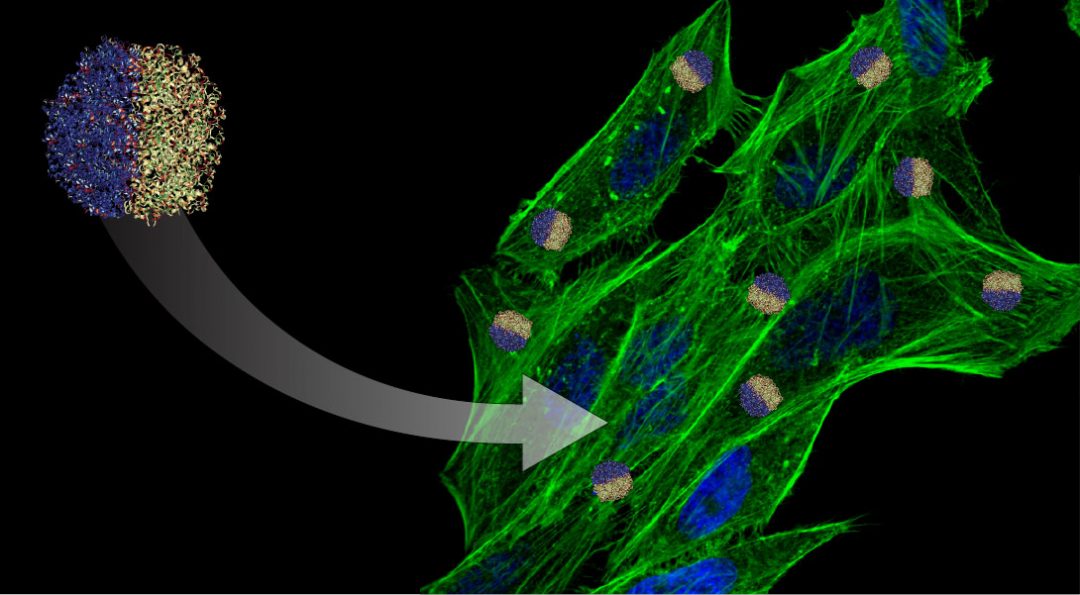
For example, the electrohydrodynamic co-jetting process, which was pioneered in the Lahann lab, permits the creation of multicompartmental particles. Independently engineering individual compartments of the nanoparticle leads to an ability to incorporate materials with orthogonal properties that may offer a solution to simultaneously address multiple biological barriers.
“Multicompartmental particles provide a set of unique features for nanoparticle targeting and controlled release of combination drugs,” said Dr. Joerg Lahann, the Wolfgang Pauli Collegiate Professor of Chemical Engineering and Director of the Biointerfaces Institute at the University of Michigan.
“While traditional nanoparticles fail to efficiently deliver the drug to target sites, our body’s circulatory cells as natural carriers of many substances have evolved properties to optimally perform delivery functions. Imparting these properties into the design of the drug delivery platforms by combining nanoparticles with circulatory cells enhances the overall outcome of the system,” added Nahal Habibi, a PhD student in the Lahann Lab working on cell-mediated drug delivery strategies.
Leukocytes are particularly good candidates because they can naturally migrate to disease-relevant regions that are often inaccessible by traditional nanoparticles, and have been used to carry therapeutic nanoparticles to cross the blood–brain barrier in a Parkinson’s disease model.
Synthetic protein nanoparticles are another emerging trend in nanomedicine.
“Advances in designing novel multicompartmental polymer/protein nanoparticles utilizing the intersection of polymer chemistry and protein biochemistry offers promise in engineering the next generation of nanoparticle formulations,” said Daniel Quevedo, another PhD candidate in Prof. Lahann’s group.
Reference: Nahal Habibi et al. ‘Emerging methods in therapeutics using multifunctional nanoparticles.’ WIREs Nanomedicine and Nanobiotechnology (2020). DOI: 10.1002/wnan.1625