A new treatment for brain cancer uses magnetic nanoparticles engineered with a specialized coating to deliver genetic material directly to tumor cells while also allowing clinicians to track them using MRI.
Brain cancers, such as medulloblastoma, are particularly daunting, not only because of the brain’s vulnerability but because tumors nestled inside are protected by the blood–brain barrier. This natural membrane shields our thinker from potentially harmful substances circulating in our blood, but also limits scientists’ ability to deliver life-saving therapies to the brain.
Tumors inside that barrier are like invaders hidden within a fortress, making them difficult to target. But research published in Advanced Science reported the development of nanoparticles that can breach the fortress walls in order to seek out the tumors while minimizing damage to essential brain cells.
“We were able to design and synthesize therapeutic and diagnostic nanoparticles tailored towards the tumor microenvironment,” said Helen Forgham from The University of Queensland’s Australian Institute for Bioengineering and Nanotechnology and one of the study’s authors.
The nanoparticles only become active on arrival at the tumor, making the treatment precise while also illuminating it in MRI scans to ultimately guide doctors and patients as they embark on treatment plans.
Breaking down barriers for brain cancer therapy
Crossing the blood–brain barrier and staying intact on the way is a significant challenge that limits current nanoparticle-led gene therapies for medulloblastoma, a hard-to-treat brain cancer prevalent in children. Existing therapies are often imprecise, causing collateral damage to other regions of the brain, or are relatively ineffective.
The nanoparticles rely on an outer shell made of fluoropolymer — a durable structure that ensures they remain stable in the bloodstream and persist long enough to reach the brain. “We were pleasantly surprised at how long our nanoparticles were able to circulate in the blood before clearance, giving them plenty of chance to reach the brain,” said Forgham.
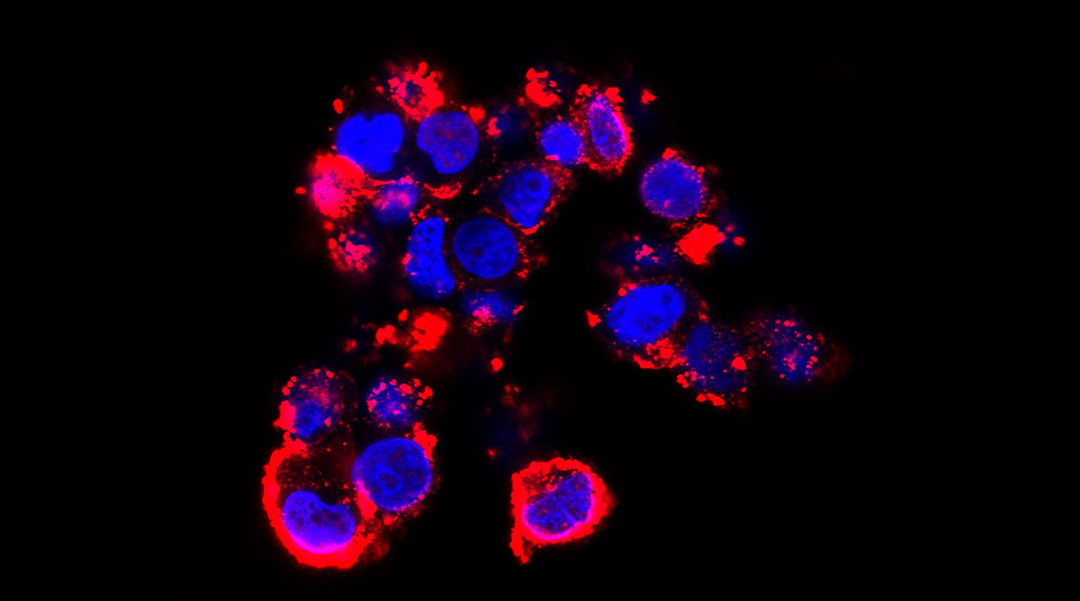
The particles could then exploit the natural transport barrier-crossing system into the brain, passively flowing in and, crucially, remaining intact. Only when the nanoparticles reach the tumor site is their full potential as an anti-cancer therapy unleashed, prompted by a change from the neutral pH in the blood to the unique environment around the tumor.
“They become highly therapeutically active at a slightly more acidic pH, as found in the tumor microenvironment,” said Forgham.
On breakdown, the nanoparticles release a genetic therapy called short interfering RNA (siRNA), which disrupts gene expression. By blocking particular genes within brain cancer cells from being expressed, it prevents protein production and slows cancer cell development. In the study, this meant slower growth of the tumors in mice.
Seeing is believing
Tracking the progress of treatment can be almost as important as the treatment itself, because complex conditions require dynamic care, promptly adjusting depending on how the tumor responds at each step. Therefore an intervention that can both treat with precision and shine a light on the progress is a unique proposition.
“As a team we have been actively involved over the years in building nanoparticles either as diagnostic probes, or as therapeutic delivery vehicles,” said one of the paper’s lead authors, Ruirui Qiao. “Here, we wanted to design dual purpose nanoparticles where the two approaches were combined for a more efficient approach. We were able to demonstrate the ability of the nanoparticles to be used as a contrast agent for MRI imaging of tumor masses in the brain.”
The hope is that these nanoparticles, and the siRNA they deliver, will work in tandem with existing treatments, so helping clinicians to watch the size, shape, and location of tumors over time will provide information about progress of the entire treatment plan, not just the nanoparticle action, though the technology isn’t quite ready for patients yet.
Looking ahead
Translation into clinics is far from straightforward, but Qiao says the team are already taking the next steps. “We are currently in our next stage of investigations,” he said. “These investigations are highly tailored towards translation. We are examining how this therapeutic approach can be combined with current chemotherapeutics with the aim of reducing drug concentrations and looking at the long-term effects of nanoparticle exposure over 6 months.”
Although cancer treatment is improving every year, most chemotherapy approaches are hard on the patients, because the toxic drugs designed to harm cancer cells invariably impact our body’s healthy ones too. Any developments that might reduce the volume or variety of drugs needed to subdue cancer would be of great value, both to patient welfare and the overall cost of care.
The team also hope the implications of this study may reach beyond this particular form of cancer. Forgham said, “Although our data is preliminary, the potential for these nanoparticles is immense not just as a potential therapeutic strategy for delivery of siRNA to medulloblastoma, but for all brain cancers.”
Reference: Thomas P. Davis, Ruirui Qiao, et al., Multifunctional Fluoropolymer-Engineered Magnetic Nanoparticles to Facilitate Blood-Brain Barrier Penetration and Effective Gene Silencing in Medulloblastoma, Advanced Science (2024). DOI: 10.1002/advs.202401340
Feature image: Nanoparticle-fluorescent siRNA uptake by D425 cells when the extracellular environment is pH 6.7. Credit: Thomas P. Davis, Ruirui Qiao, et al.