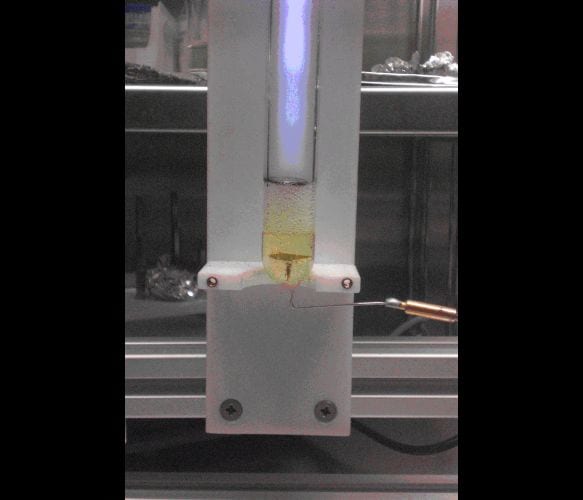
More than one hundred years ago J. Gubkin ignited a direct current glow discharge plasma in the gas volume of a glass bulb, half-filled with an aqueous solution of silver nitrate. One electrode was placed in the solution, the other one was located above the solution surface in the gas phase. At a voltage of roughly 1000 V a glow discharge plasma was created, leading to the formation of silver on the solution surface as a result of the interaction of the silver cations at the free surface of the electrolyte with the electrons from the plasma.
More than one hundred years ago J. Gubkin ignited a direct current glow discharge plasma in the gas volume of a glass bulb, half-filled with an aqueous solution of silver nitrate. One electrode was placed in the solution, the other one was located above the solution surface in the gas phase. At a voltage of roughly 1000 V a glow discharge plasma was created, leading to the formation of silver on the solution surface as a result of the interaction of the silver cations at the free surface of the electrolyte with the electrons from the plasma.
The plasma-electrochemical approach has hitherto been the only electrochemical route to the synthesis of free nanoparticles. Due to the high vapour pressure of water, atmospheric-pressure microplasmas or plasma jets are nowadays used to generate colloidal metal nanoparticles. In aqueous media this method is restricted to the generation of noble metal particles due to the limited electrochemical window of water. Ionic liquids, due to their extremely low vapour pressures and wide electrochemical windows, are well suitable electrolytes for the plasma electrochemical process and in addition the synthesis of nanoparticles of reactive metals is possible. In the presented article German researchers show that copper nanoparticles with sizes of below 10 nm can be made easily with a glow discharge argon plasma above a solution of CuCl and CuCl2 dissolved in the ionic liquid 1-butyl-3-methylimidazolium dicyanamide ([BMIm]dca), respectively. This approach opens the way for the production of reactive metal and semiconductor nanoparticles.